Generic Drugs Market: What You Need to Know About Safety, Cost, and Real-World Results
When you pick up a generic drugs market, the collection of lower-cost versions of brand-name medications approved by regulators like the FDA. Also known as non-branded medications, it makes up over 90% of prescriptions filled in the U.S. and saves patients and insurers billions each year. But here’s the thing: just because it’s cheaper doesn’t mean it’s always the same. Real-world data shows some generics work just fine, while others trigger unexpected side effects—especially with heart meds, thyroid pills, and seizure drugs.
The cardiovascular generics, generic versions of drugs used to treat high blood pressure, heart failure, and arrhythmias are one of the biggest areas where differences show up. A patient switching from a brand-name beta-blocker to a generic might feel fine—or they might get dizzy, fatigued, or have their heart rate spike. Studies tracking thousands of users found that while most generics perform as expected, a small but significant number cause problems in people with narrow therapeutic windows. That’s why keeping a medication journal, a personal log tracking how your body reacts to each drug change is critical. Write down sleep patterns, energy levels, or weird symptoms after switching. That journal could be the key to catching a bad batch before it hurts you.
Then there’s the contamination issue. Recent recalls have pulled generic drug contamination, the presence of harmful chemicals like NDMA or benzene in otherwise approved medications from shelves. These aren’t random errors—they’re linked to manufacturing shortcuts in some overseas labs. Benzene in Mucinex generics. NDMA in blood pressure pills. These aren’t scare stories. They’re FDA alerts. If you take a generic daily, know what you’re on. Check the FDA’s recall page. Ask your pharmacist if the batch you got has been flagged.
Not all generics are created equal, and not all patients react the same. levothyroxine generics, generic versions of the thyroid hormone replacement drug are a perfect example. For most people, switching brands won’t matter. But if you have thyroid cancer, heart disease, or a history of bad reactions, even a tiny change in absorption can throw your whole system off. That’s why your doctor might tell you to get a TSH test 6 to 8 weeks after any switch. It’s not overkill—it’s smart.
The generic drugs market isn’t just about saving money. It’s about knowing when to trust it—and when to dig deeper. The posts below cover real cases: people who got sick after switching heart meds, others who caught contamination early thanks to a journal, and patients who found safer alternatives when generics failed. You’ll see how probiotics help when antibiotics knock out your gut, how citrus fruits mess with blood pressure pills, and why some antidepressants increase bleeding risk. This isn’t theory. It’s what’s happening in clinics, pharmacies, and homes right now. What you read here could help you avoid a dangerous mistake—or save your life.
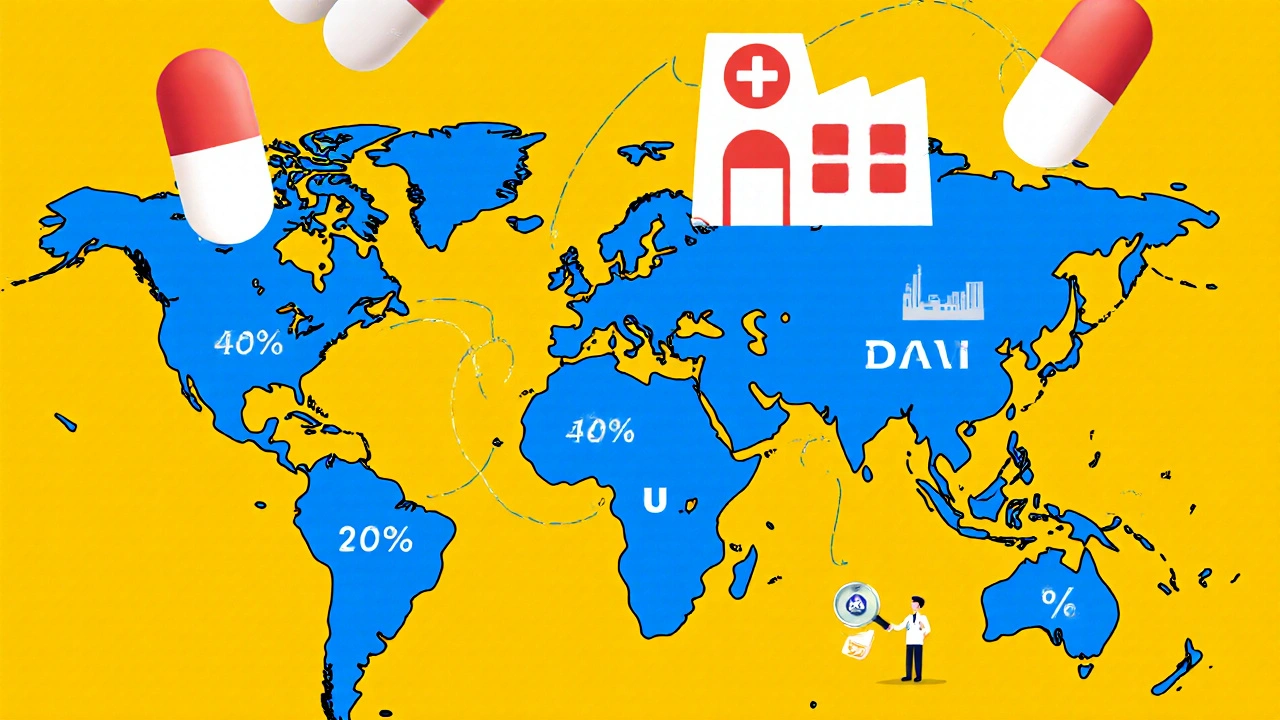
Future of Global Generic Markets: Key Predictions and Trends Through 2030
Generic drugs save billions in healthcare costs worldwide. Discover the key trends shaping their future: biosimilars, supply chain risks, quality control, and which countries are leading the market through 2030.
View More