When a pharmacist sees a brand-name prescription, they don’t just fill it-they evaluate whether a generic version could work just as well, often saving the patient hundreds of dollars a year. But changing a prescription isn’t as simple as swapping labels. It requires clear, evidence-based communication with the prescriber. This isn’t about cutting costs alone; it’s about making sure the patient gets the right medicine safely and consistently.
Why Generics Matter-And Why Prescribers Hesitate
Nearly 97% of prescriptions filled in the U.S. are for generic drugs. That’s not a coincidence. The FDA’s Orange Book, updated annually, lists over 12,800 generic products that are therapeutically equivalent to their brand-name counterparts. These generics save the healthcare system about $409 billion every year. Yet despite the data, many prescribers still hesitate to allow substitutions. A 2023 survey found that 37.6% of prescribers worry generics might not work as well, especially for complex drugs like inhalers or topical creams. Some believe patients respond differently. Others just don’t know the science behind bioequivalence. The truth? Generic drugs must meet the same strict standards as brand-name drugs. The FDA requires that the active ingredient match exactly, and the body must absorb it within a very tight range-90% of the time, absorption is within 95% to 105% of the brand. That’s tighter than most clinical variations. So why the hesitation? Often, it’s lack of information. Pharmacists who explain the data clearly-citing specific Orange Book ratings, bioequivalence ranges, or real-world adherence studies-see much higher acceptance rates.The Rules: What Pharmacists Can and Can’t Do
Every state has its own rules about generic substitution. In 49 states, pharmacists can substitute a generic unless the prescriber writes “dispense as written” (DAW). That happens on about 15.3% of prescriptions. In 17 states, pharmacists must get the patient’s consent before switching. Five states-Connecticut, Massachusetts, New York, Texas, and Virginia-only allow substitution if the generic is on a state-approved formulary. The FDA’s Orange Book is the bible here. Each drug gets an “A” or “B” rating. “A” means therapeutically equivalent. “B” means it’s not. Over 92% of generics are rated “A.” But here’s the catch: not all “A” ratings are equal. For drugs with a narrow therapeutic index-like warfarin, levothyroxine, or phenytoin-switching even between two approved generics can cause problems. These drugs have a tiny window between effective and toxic doses. Pharmacists must flag these cases and talk to the prescriber before any change.When Communication Is Non-Negotiable
There are specific red flags that demand a call to the prescriber:- Narrow therapeutic index (NTI) drugs: Even small changes in blood levels can cause harm. Substitution here requires explicit approval.
- Excipient allergies: Generics can have different fillers, dyes, or preservatives. About 8.7% of substitution issues come from these inactive ingredients. A patient allergic to lactose or a specific dye needs a tailored solution.
- DAW prescriptions: If the prescriber wrote “do not substitute,” pharmacists must honor it-but they can still ask why. Sometimes it’s based on outdated info. A quick call with data can change that.
- Complex formulations: Extended-release tablets, transdermal patches, or inhalers have delivery mechanisms that matter. While generics are approved, prescribers may be unfamiliar with the data. Pharmacists need to bring the Product-Specific Guidance (PSG) from the FDA to the conversation.
How to Talk to Prescribers-And Get Them to Listen
A 2021 study showed that pharmacists using a structured approach got prescribers to agree to generic substitution 82.4% of the time. Those who just said, “This generic is fine,” got agreement only 57.3% of the time. The difference? Structure. Here’s what works:- Call within 24 hours. Don’t wait. The sooner you reach out, the less likely the prescription is filled incorrectly.
- Use the Orange Book. Say: “This generic has an ‘A’ rating per the FDA’s 2023 Orange Book. The bioequivalence data shows absorption within 98% of the brand.”
- Include cost data. “This generic saves the patient $120 a month. That’s $1,440 a year.”
- Share adherence data. “A 2018 study of 12.7 million patients showed 12.4% better adherence with generics-and 28.6% fewer hospitalizations.”
- Document everything. Use the EHR. Note the time, method, prescriber name, and reason. CMS requires this for Medicare Part D, and pharmacies using digital systems hit 98.7% compliance.
Barriers-and How to Overcome Them
The biggest obstacles aren’t legal or technical. They’re human. - Time: Pharmacists are stretched thin. AI tools like PharmAI’s Generic Substitution Assistant now help by auto-pulling FDA data and drafting messages. Pharmacies using these tools report 42% faster communication and 94.2% accuracy. - Knowledge gaps: One in two pharmacists feels unsure about modified-release generics. That’s why the FDA runs free “Orange Book Live” Q&As every quarter. Pharmacist-led webinars are now part of continuing education. - Prescriber resistance: 58.3% of doctors cite therapeutic equivalence concerns. But when pharmacists give them a single slide with bioequivalence ranges and adherence stats, acceptance jumps by over 34 percentage points.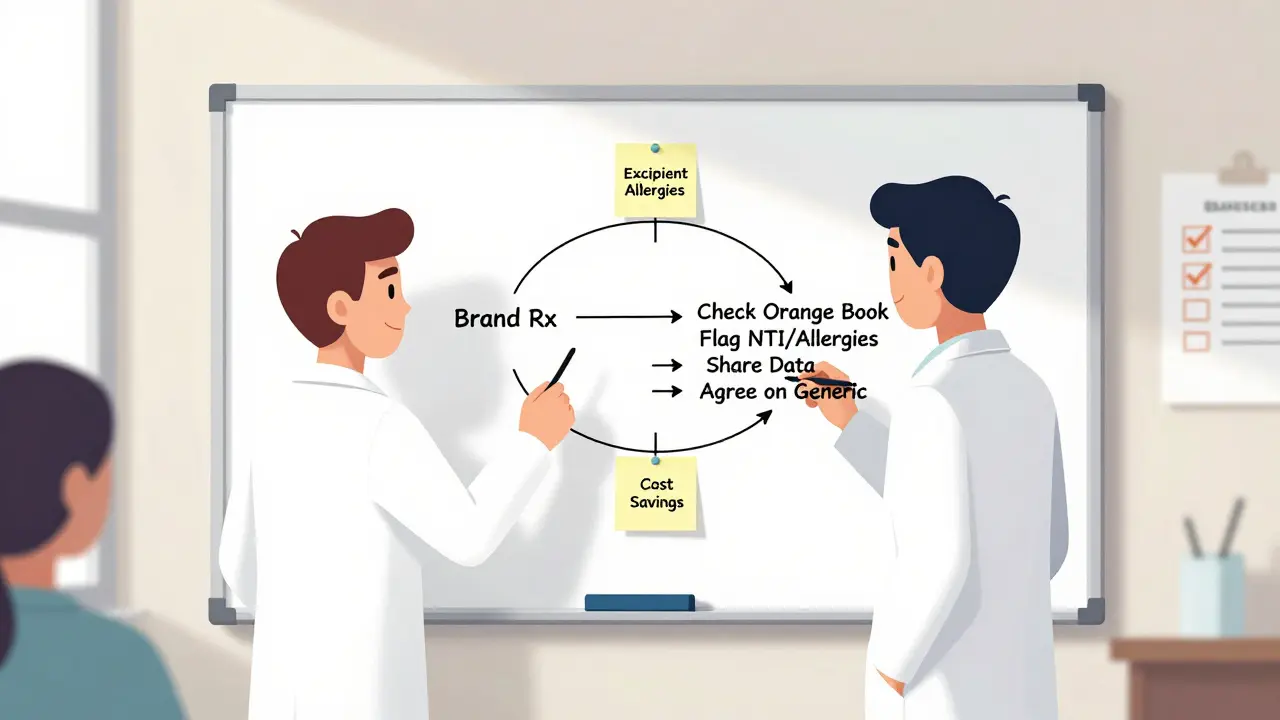
The Bigger Picture: Value-Based Care and New Rules
This isn’t just about saving money anymore. It’s about better outcomes. Since January 2025, the Inflation Reduction Act has expanded Medicare Part D’s medication therapy management (MTM) services. Pharmacists are now formally recognized as key players in optimizing drug regimens-especially for seniors on multiple generics. Over 63% of accountable care organizations (ACOs) now track generic substitution rates as a quality metric. The CDC is launching a Generic Medication Safety Network in mid-2024. It will give pharmacists near-real-time alerts if a generic version shows unexpected side effects in the field. That’s a game-changer.What Good Documentation Looks Like
Good documentation isn’t just compliance. It’s protection. It’s proof that you did your job right. The AMA and APhA recommend including these five elements in every communication:- Date and time of contact
- Method used (phone, secure message, EHR portal)
- Name and credentials of the prescriber
- Specific recommendation and why (e.g., “Recommended generic with A rating due to equivalent bioequivalence and $98 monthly savings”)
- Outcome: Did the prescriber agree? Was the prescription changed?
Final Thought: It’s Not About Substitution. It’s About Partnership.
Pharmacists aren’t trying to override doctors. They’re trying to support better care. Generics aren’t cheaper because they’re lower quality. They’re cheaper because the patent expired-and the science hasn’t changed. The best pharmacists don’t just fill prescriptions. They bridge the gap between clinical guidelines and real-world practice. They turn a simple substitution into a conversation that improves adherence, cuts costs, and protects patients. When a pharmacist calls a prescriber to recommend a generic, they’re not asking for permission. They’re offering evidence. And when prescribers listen, everyone wins.Can pharmacists switch a brand-name drug to a generic without the doctor’s permission?
In 49 states, yes-if the prescription doesn’t say “dispense as written” and the generic has an FDA-approved “A” rating. But pharmacists must still check for exceptions like narrow therapeutic index drugs or patient allergies. In 17 states, patient consent is also required. Five states only allow substitution from a state-approved list of generics.
Are generics really as safe and effective as brand-name drugs?
Yes. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand. They must also prove bioequivalence-meaning the body absorbs them within 80%-125% of the brand. Real-world data shows 98.7% of approved generics meet this within 95%-105%. Studies of over 12 million patients found no difference in effectiveness or safety.
Why do some prescribers refuse generic substitution?
Common reasons include concerns about therapeutic equivalence (especially for complex drugs like inhalers or extended-release tablets), fear of patient complaints, or outdated beliefs. Some have seen a patient react differently after a switch, though this is rare. Pharmacists can overcome this by sharing FDA bioequivalence data, adherence studies, and cost savings-turning assumptions into evidence.
What should I do if my pharmacist recommends a generic but I’m worried?
Ask your pharmacist to explain why they’re recommending it. Request the FDA’s Orange Book rating and the bioequivalence data. You can also ask if the generic has the same inactive ingredients as your previous brand. If you still feel unsure, ask your prescriber for a second opinion. Most prescribers appreciate patients who ask questions.
Do generic drugs cause more side effects?
No. Side effects come from the active ingredient, which is identical in generics and brand-name drugs. However, inactive ingredients (like dyes or fillers) can differ and may cause reactions in patients with allergies. About 1 in 12 patients with known sensitivities experience issues from these differences. Always tell your pharmacist about any allergies to dyes, lactose, or preservatives.
How does the FDA ensure generics are equivalent?
The FDA requires generics to undergo rigorous testing. Manufacturers must prove their product delivers the same amount of active ingredient into the bloodstream at the same rate as the brand. This is measured using AUC and Cmax values, with a 90% confidence interval between 80% and 125%. In practice, most generics fall within 95%-105%, meaning they’re nearly identical in how the body uses them.
What’s the difference between “A” and “B” ratings in the Orange Book?
“A” means the generic is therapeutically equivalent to the brand-same active ingredient, same performance, and interchangeable. “B” means it’s not equivalent. This could be due to differences in formulation, delivery, or lack of bioequivalence data. Pharmacists should never substitute a “B” rated product without prescriber approval.
Can I ask my pharmacist to stick with the brand name?
Yes. You can always ask to keep your brand-name drug, even if a generic is available. Some patients prefer the brand for consistency, especially with chronic conditions. Your pharmacist can help you understand the cost difference and may help you apply for financial assistance if the brand is too expensive.