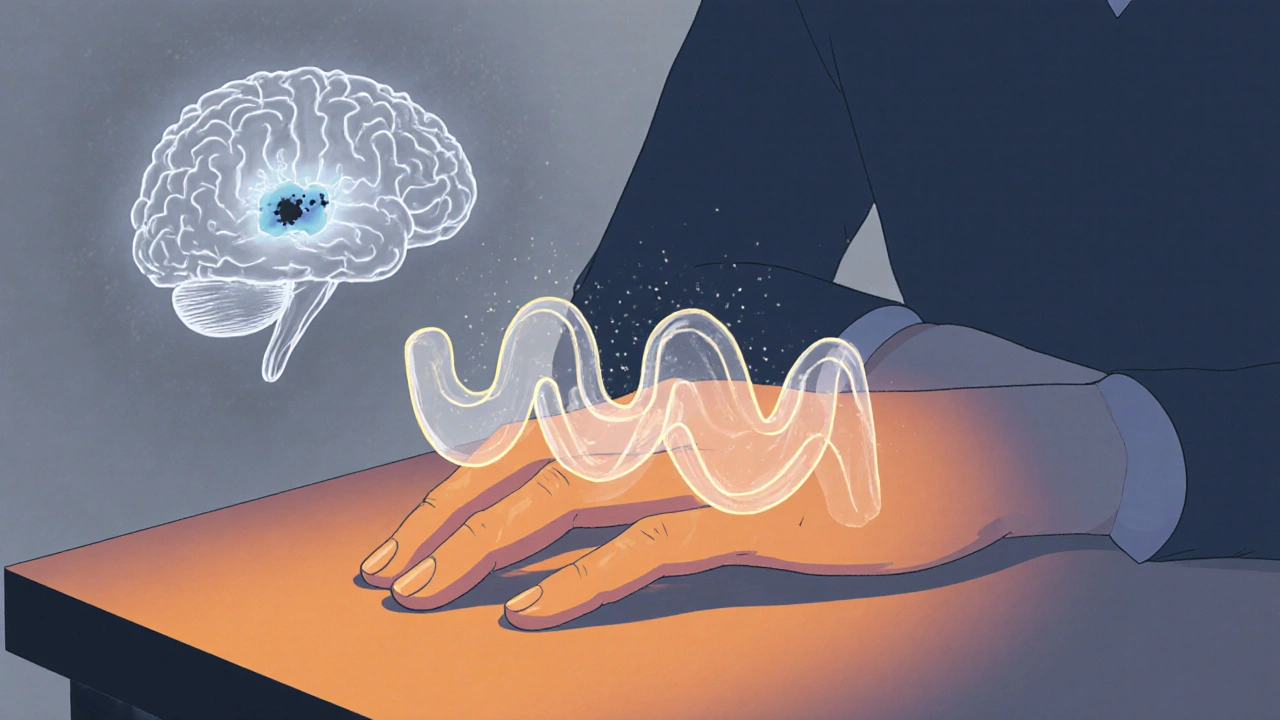
When your hand starts shaking for no reason - not from caffeine or cold, but a quiet, rhythmic tremor that only shows up when you're resting - it’s easy to brush it off. Maybe it’s stress. Maybe you’re tired. But if that tremor sticks around, and your movements feel slower, your muscles tighter, like they’re wrapped in invisible bands, it could be something deeper. For over 10 million people worldwide, this isn’t just a passing quirk. It’s Parkinson’s disease.
What Happens in the Brain?
Parkinson’s isn’t just about shaking. It’s about a slow, silent loss inside the brain. A small group of nerve cells in the substantia nigra - the part that makes dopamine - begins to die off. By the time symptoms show up, about 60 to 80% of those cells are already gone. Dopamine is the brain’s natural signal for smooth, controlled movement. Without enough of it, the circuits that tell your muscles when and how to move start to misfire. This isn’t a sudden breakdown. It’s a quiet erosion. You don’t wake up one day unable to walk. You notice you’re not swinging your arm when you walk anymore. Your handwriting gets smaller. Buttoning a shirt takes longer. Then comes the tremor - often starting in just one hand. A slow, side-to-side motion, like rolling a pill between your thumb and finger. It’s called a pill-rolling tremor. And it’s most noticeable when you’re not moving. Sit still, and it’s there. Pick up a cup, and it fades.The Stiffness That Doesn’t Go Away
Tremor gets attention, but stiffness - or rigidity - is just as disabling. It’s not the kind of tightness you feel after a workout. This is constant. Your arm or leg feels heavy, resistant, like moving through thick syrup. Doctors describe it as cogwheel rigidity - a jerky, ratcheting resistance when they move your limb during an exam. Or lead-pipe rigidity - a smooth, unyielding tightness. This stiffness isn’t just inconvenient. It causes pain. It makes it hard to turn in bed. To stand up from a chair. To write a note or tie your shoes. A study from Parkinson’s UK found that 73% of people with Parkinson’s struggle with these everyday tasks within three years of diagnosis. And it’s not just the arms. The neck stiffens. The back curves. The face loses expression - a blank stare called masked facies. This rigidity happens because dopamine loss throws off the balance between two pathways in the basal ganglia - the brain’s movement control center. One pathway says “go,” the other says “stop.” Without dopamine, the stop signal wins. Your muscles stay tense. Movement becomes effortful.Dopamine Replacement: The Lifeline - and the Trade-Off
There’s no cure for Parkinson’s. But there is a treatment that changes lives: dopamine replacement. The gold standard is levodopa, often paired with carbidopa. Levodopa is a chemical your body turns into dopamine. Carbidopa stops it from breaking down before it reaches the brain, so more gets where it’s needed. This combo - sold as Sinemet, Rytary, or generic levodopa/carbidopa - works fast. Most people feel better within 30 to 60 minutes. For the first few years, it’s almost miraculous. You move again. You smile. You write. You walk without dragging your feet. Studies show up to 70% improvement in motor symptoms during this early “honeymoon” phase. But it doesn’t last forever. After five to ten years, the magic starts to fade. The same dose that gave you six hours of smooth movement now only gives you two. You start having “off” periods - sudden, unpredictable drops in mobility. Then come the “on” periods - but not the good kind. Instead of smooth movement, you get dyskinesias: involuntary, dance-like movements. Arms flail. Legs jerk. It’s not the disease itself - it’s the medicine.Why Does This Happen?
The brain’s dopamine system becomes less stable over time. As more cells die, the brain loses its ability to store and release dopamine naturally. Levodopa works by flooding the system, but without healthy nerve cells to regulate it, the levels spike and crash. That’s why you get the swings - too much dopamine one minute, too little the next. It’s not just about the dose. What you eat matters too. Protein - from meat, dairy, eggs - competes with levodopa for the same transporters into the brain. A big steak at lunch can make your afternoon dose useless. Many people learn to take levodopa 30 to 60 minutes before meals. Others shift protein to dinner.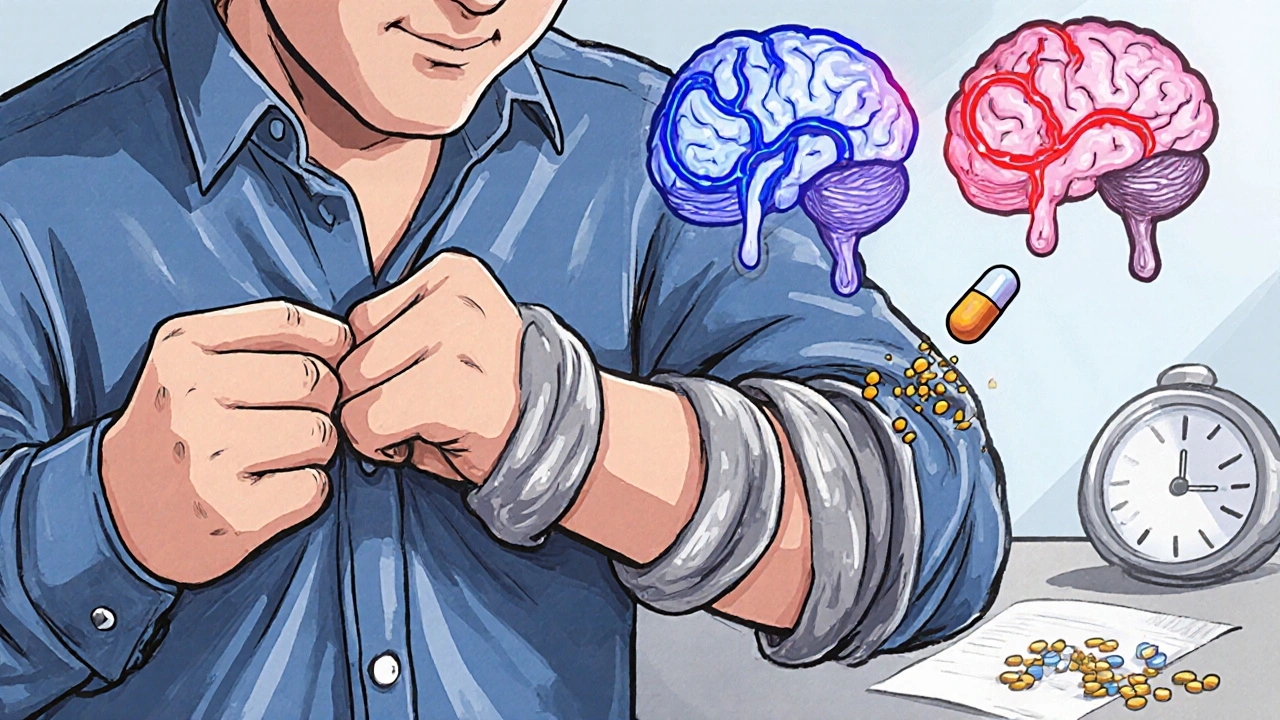
Alternatives to Levodopa
Not everyone starts with levodopa. For younger patients, or those with milder symptoms, doctors often begin with dopamine agonists like pramipexole or ropinirole. These drugs mimic dopamine by binding directly to its receptors. They’re about 30 to 50% as effective as levodopa for movement, but they cause fewer early dyskinesias. The trade-off? More side effects like dizziness, nausea, sleepiness, and sometimes impulse control issues - gambling, shopping, or hypersexuality. A 2023 review from the Parkinson’s Foundation found that 60% of patients eventually need both an agonist and levodopa to stay functional. There are also newer options. Inbrija, an inhaled form of levodopa, can rescue you during an “off” episode in under 10 minutes. But it costs $3,700 a month. Extended-release pills like Rytary cut dosing from three to two times a day - but cost $5,800 a year, compared to $600 for the generic version.Real People, Real Challenges
Online forums are full of stories like these: - “I’ve been on carbidopa/levodopa for eight years. My ‘on’ time used to be six hours. Now it’s two. And the dyskinesias? They’re worse than the stiffness.” - ParkinDad, Parkinson’s Foundation community - “I started pramipexole at diagnosis. Five years later, I’m still driving, cooking, hiking. No dyskinesias. Just tired sometimes.” - SilverLining2022 A survey from the Michael J. Fox Foundation found that 56% of people say timing their meds is the hardest part of daily life. And 72% say “wearing-off” episodes ruin their ability to do chores, work, or even talk to family.How Doctors Adjust Treatment Today
The old rule - start levodopa early and go hard - is gone. Experts now use a “start low, go slow” approach. Begin with 25/100 mg once or twice a day. Increase slowly, only if needed. This reduces nausea, dizziness, and the risk of early dyskinesias. A 2022 study in the Journal of Parkinson’s Disease showed that starting levodopa early doesn’t speed up the disease. But for someone under 60, delaying it by a year or two can delay the worst side effects by several years. Doctors now treat Parkinson’s like a puzzle - not a switch. It’s about balancing movement, side effects, quality of life, and daily routine. Some people need three doses a day. Others need five. Some take pills with water. Others use liquid formulations.
What’s Next?
Researchers are working on better ways to deliver dopamine. One promising method is a continuous infusion under the skin - a pump that slowly releases levodopa all day. A 2022 trial showed it added 2.5 more “on” hours per day compared to pills. But it’s still experimental and not widely available. Gene therapies and stem cell treatments are in early testing. So are drugs that protect remaining dopamine cells - not just replace what’s lost. For now, dopamine replacement remains the most powerful tool we have. It doesn’t stop Parkinson’s. But it lets people live with it - longer, better, and with more dignity.Managing Medication Daily
Taking Parkinson’s meds isn’t like taking a vitamin. It’s a full-time job. The average person spends 15 minutes a day managing their pills early on. By mid-stage, that jumps to 45 minutes. Nearly 80% need help from a caregiver to track doses, meals, and timing. Tips that help:- Use a pill organizer with alarms
- Take levodopa 30-60 minutes before meals high in protein
- Keep a symptom and medication log - note when you feel “on” or “off”
- Don’t skip doses, even if you feel fine - skipping can trigger sudden “off” episodes
- Ask your doctor about extended-release options if you’re taking pills four or five times a day
It’s exhausting. But small routines make a big difference.
It’s Not Just About Movement
Parkinson’s affects more than muscles. Depression, sleep problems, constipation, and memory changes are common. Dopamine replacement helps movement - but not these. That’s why a full care team matters: neurologist, physical therapist, dietitian, counselor. You don’t have to manage this alone. Support groups, online communities, and movement disorder specialists can help you find the right balance - between meds and meals, movement and rest, hope and reality.Is Parkinson’s disease caused by low dopamine?
Yes. Parkinson’s is caused by the loss of dopamine-producing nerve cells in a part of the brain called the substantia nigra. By the time tremors or stiffness appear, 60-80% of these cells are already gone. Dopamine helps control movement, so when levels drop, motor symptoms like shaking and stiffness begin.
Can you cure Parkinson’s with dopamine replacement?
No. Dopamine replacement, like levodopa, only treats symptoms - it doesn’t stop the brain cells from dying. It helps people move better and live more normally, but it doesn’t cure the disease. Over time, the brain’s ability to use dopamine declines, and side effects like dyskinesias can develop.
Why does levodopa stop working after a few years?
As more dopamine cells die, the brain loses its ability to store and release dopamine naturally. Levodopa floods the system, but without healthy cells to regulate it, levels spike and crash. This leads to “wearing-off” periods and unpredictable “on-off” swings. The brain also becomes more sensitive to dopamine, causing involuntary movements called dyskinesias.
Do protein-rich meals affect levodopa?
Yes. Protein uses the same transport system as levodopa to enter the brain. A large meal with meat, cheese, or eggs can block levodopa absorption, making it less effective. Many people take levodopa 30-60 minutes before meals or shift protein to the evening to avoid this.
Are dopamine agonists better than levodopa?
Not necessarily. Dopamine agonists like pramipexole are less effective at controlling movement than levodopa - about 30-50% as strong. But they cause fewer early dyskinesias, so they’re often used first in younger patients. Most people eventually need both. The choice depends on age, symptoms, side effects, and lifestyle.
How often should Parkinson’s medication be adjusted?
Medication needs change as the disease progresses. Early on, doses may be adjusted every few weeks. Later, changes may be needed every few months - or even monthly - as “wearing-off” and dyskinesias appear. Regular check-ins with a neurologist or movement disorder specialist are key. Don’t wait for a crisis to adjust your plan.
8 Comments
Lisa Detanna
November 24 2025
I work with a lot of Parkinson’s patients in rehab. The thing nobody talks about? The shame. They don’t want to eat in public because their hands shake. They avoid hugs because their arms won’t relax. And when the dyskinesias hit? They’ll sit in the car for 20 minutes before walking into the store. It’s not just a disease-it’s a social prison.
Demi-Louise Brown
November 26 2025
Consistency is critical. Taking levodopa at the same time every day, away from protein, creates predictability. Predictability reduces anxiety. Anxiety exacerbates symptoms. A structured routine, even if it feels robotic, restores a sense of control. Many patients report improved quality of life not from new drugs, but from disciplined timing.
Matthew Mahar
November 27 2025
OMG I JUST REALIZED MY DAD HAS BEEN DOING THE PILL ROLLING THING FOR YEARS AND I THOUGHT HE WAS JUST NERVOUS LOL. HE’S 68. I’M GOING TO MAKE HIM SEE A DOCTOR TOMORROW. THANK YOU FOR THIS POST. I DIDN’T KNOW ANY OF THIS.
John Mackaill
November 28 2025
I’ve been on pramipexole for seven years. No dyskinesias. Just constant fatigue. But I still hike. Still cook. Still dance with my wife on Sundays. It’s not perfect. But it’s enough. For me, the trade-off was worth it. I’d rather be tired than twitching.
Adrian Rios
November 29 2025
Let me tell you something nobody says out loud: levodopa isn’t just medicine-it’s a daily negotiation with your own body. You learn to read your own limbs like a weather forecast. Morning: clear skies, full mobility. Afternoon: sudden thunderstorm, legs locked. Evening: fog, brain fog, can’t remember your own phone number. And you start to wonder-am I still me, or am I just the sum of my dopamine levels? It’s not just a neurological disease. It’s an existential one.
Casper van Hoof
December 1 2025
The philosophical paradox of Parkinson’s treatment lies in its temporal asymmetry: dopamine replacement alleviates motor symptoms while simultaneously accelerating the phenomenological alienation of the self. The very agent that restores agency-levodopa-becomes the instrument of its eventual erosion through dyskinesia and wearing-off. Thus, the cure becomes a mirror of the disease’s inherent temporality.
Richard Wöhrl
December 3 2025
One thing I wish more people knew: protein doesn’t just interfere with levodopa-it can completely block it. I had a patient who was taking her meds with breakfast, then complaining her meds weren’t working. Turned out she was eating scrambled eggs, bacon, and yogurt every morning. I told her to try a smoothie with banana and almond butter instead. Within two weeks, her 'on' time doubled. Small changes. Huge impact. Also-use a pill organizer with alarms. I swear by it. I’ve seen people who forget one dose spiral into a week of 'off' time. It’s brutal.





Laurie Sala
November 23 2025
My mom’s been on levodopa for 12 years… and I swear, some days she’s a completely different person. One minute she’s laughing, buttoning her coat, typing a text… the next, she’s frozen, drooling, just staring at the wall. It’s like watching someone get switched on and off with a remote. I don’t know which is worse-the shaking or the silence.