
Every time you pick up a prescription and see a cheaper version of a brand-name drug, you’re holding the result of something called an ANDA. That’s short for Abbreviated New Drug Application. It’s not a fancy term, but it’s one of the most powerful tools in American healthcare - responsible for saving patients and insurers over $300 billion a year. And yet, most people have never heard of it.
What Exactly Is an ANDA?
An ANDA is a request submitted to the U.S. Food and Drug Administration (FDA) to get approval to sell a generic version of a brand-name drug. It’s called “abbreviated” because it doesn’t require the same long, expensive clinical trials that the original drug had to go through. Instead, the company making the generic version just needs to prove their product works the same way - same active ingredient, same strength, same way it’s taken - and that your body absorbs it at the same rate and amount.
This process was created by the Hatch-Waxman Act of 1984. Before that, generic drugs were stuck in legal and regulatory limbo. Brand-name companies held patents that blocked anyone else from making copies, even after the patent expired. The Hatch-Waxman Act changed that. It gave generic manufacturers a clear path to enter the market once patents expired - but only if they could prove their drug was just as safe and effective.
How Does an ANDA Work?
Here’s how it actually works in practice:
- The generic drug maker picks a brand-name drug already approved by the FDA - this is called the Reference Listed Drug (RLD). You can find these in the FDA’s Orange Book, which lists all approved drugs and their patent status.
- They make a copy of that drug. It doesn’t have to look the same - the color, shape, or inactive ingredients can be different - but the active ingredient must be identical.
- They run bioequivalence studies. These are small clinical trials with 24 to 36 healthy volunteers. Blood samples are taken over time to measure how much of the drug enters the bloodstream and how fast. The generic must deliver the same amount of drug within a tight range - between 80% and 125% of the brand-name version.
- They submit the ANDA. This includes detailed information on how the drug is made, how quality is controlled, and how stable it is over time. Everything must match FDA standards.
- The FDA reviews it. Under current rules, they have 10 months to decide. If everything checks out, they give it an ANDA number - like ANDA 214,455 - and the generic hits the market.
That’s it. No testing on thousands of patients. No years of Phase 1, 2, and 3 trials. Just science-backed proof that it does the same job.
ANDA vs. NDA: The Big Difference
Every new brand-name drug starts with a New Drug Application, or NDA. That’s the full, expensive route. Companies spend 10 to 15 years and over $2 billion to develop an NDA. They test the drug in animals, then in small groups of people, then in large clinical trials to prove it works and is safe.
An ANDA skips all that. It’s not a shortcut because it’s lazy - it’s a shortcut because the science already exists. The FDA has already approved the original drug. The question isn’t whether the drug works - it’s whether the copy works just as well.
That’s why generic drugs cost 80% to 85% less than brand-name versions. The savings aren’t from cutting corners. They’re from not repeating what’s already been proven.
What Gets Approved? What Doesn’t?
Not every drug can go through the ANDA process. It works best for simple, small-molecule pills - things like blood pressure meds, antibiotics, or cholesterol drugs. These have clear chemical structures and predictable absorption in the body.
But it gets tricky with complex products:
- Generic inhalers - the way the drug is sprayed matters. Tiny differences in the propellant or nozzle can change how much reaches the lungs.
- Topical creams - how the drug penetrates the skin isn’t easy to measure with blood tests.
- Drugs with narrow therapeutic windows - like warfarin or lithium - where even a small change in dose can cause serious side effects.
For these, the FDA has been working on new guidance since 2022 to help manufacturers prove bioequivalence. But it’s still harder. Many of these complex generics are still waiting for approval.
Who Uses ANDAs - And Why It Matters
Over 90% of all prescriptions filled in the U.S. are for generic drugs. That’s not because doctors are pushing cheaper options - it’s because patients choose them. And the reason they’re available? ANDAs.
Companies like Teva, Mylan (now Viatris), and Sandoz are the main players. They spend millions developing ANDAs, knowing that once a patent expires, they can flood the market with low-cost versions. The first company to file a successful ANDA for a popular drug often gets 180 days of exclusive rights to sell it - which is why you’ll sometimes see multiple generic versions appear all at once.
The numbers are staggering. In 2022 alone, the FDA approved 724 generic drugs. Those approvals are projected to save the U.S. healthcare system $23.7 billion in one year. Since 1984, generic drugs have saved over $2.2 trillion.
And it’s not just about money. It’s about access. A diabetic who can’t afford insulin today might be able to afford it tomorrow - if a generic version gets approved through an ANDA.
Common Problems With ANDA Submissions
Getting an ANDA approved isn’t easy. Even with a clear path, many applications get rejected. The FDA sends out “complete response letters” when something’s missing.
The top three reasons ANDAs get turned down:
- Manufacturing issues - 32% of rejections. The facility doesn’t meet quality standards. The process isn’t consistent. The drug might be fine, but if it’s made in a dirty or poorly controlled environment, it won’t pass.
- Inadequate bioequivalence data - 27% of rejections. The study didn’t prove the drug behaves the same way in the body. Maybe the sample size was too small, or the timing of blood draws was off.
- Patent certification errors - Companies must declare how they’re handling every patent listed for the brand-name drug. Mess this up, and the FDA can’t approve it until the legal issues are sorted.
Smaller companies struggle the most. A 2022 survey found that 68% of small generic manufacturers had trouble with complex bioequivalence studies. On average, each ANDA submission gets two or three rejection letters before it’s approved.
What’s Changing in 2026?
The FDA is pushing to make the ANDA process faster and more reliable. Under the new GDUFA IV rules (2023), they’re aiming for 90% of original ANDAs to be approved on the first try by 2027. Right now, it’s about 65%.
They’re also expanding the types of drugs that can use the ANDA pathway. Complex generics - like nasal sprays, injectables, and transdermal patches - are getting more attention. By 2028, experts predict that 25% of all generic drugs will be complex products, up from 15% today.
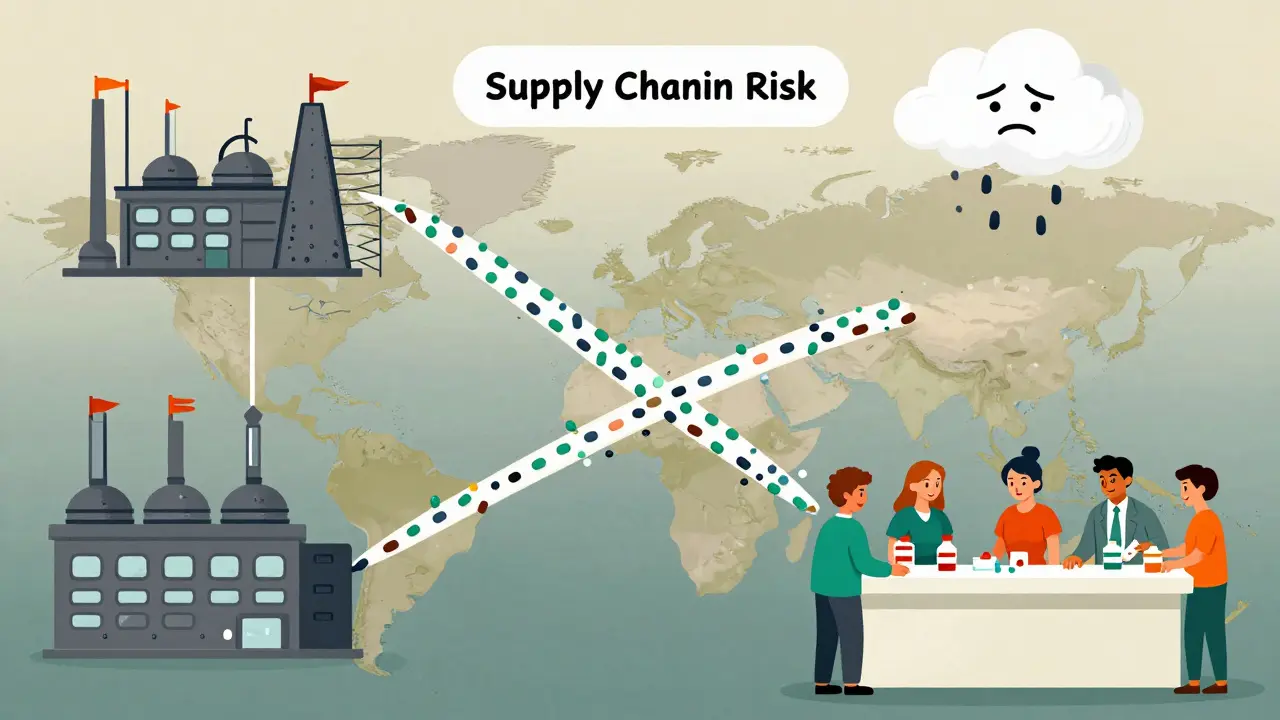
But there’s a warning. Over 80% of generic drug ingredients come from just two countries: India and China. If supply chains break down - because of weather, politics, or a pandemic - drug shortages can happen fast. The FDA is working on diversifying suppliers, but it’s a slow process.
Why This Matters to You
You don’t need to file an ANDA. But you benefit from it every time you fill a prescription.
If you’re on a fixed income, an ANDA-approved generic could mean the difference between taking your medication or skipping doses. If you’re covered by insurance, it keeps premiums lower. If you’re a taxpayer, it reduces spending on Medicare and Medicaid.
And if you ever wonder why your pill looks different from last time - it’s not a mistake. It’s probably a different generic manufacturer. The active ingredient is the same. The effect is the same. The price? Much lower.
The ANDA system isn’t perfect. It’s slow for complex drugs. It’s vulnerable to global supply issues. But it’s also one of the most successful public health policies ever written. It doesn’t just lower prices - it saves lives by making medicine affordable.
Is an ANDA the same as a generic drug?
No. An ANDA is the application submitted to the FDA to get approval for a generic drug. The generic drug is the actual medicine you take. The ANDA is the paperwork that proves it’s safe and effective.
Can any company file an ANDA?
Yes, any company can file - but it’s not easy. They need expertise in pharmaceutical development, regulatory affairs, and manufacturing. Most successful filers have teams of scientists and regulatory specialists who’ve done this before. Small companies often partner with consultants or outsource parts of the process.
How long does it take to get an ANDA approved?
The FDA has 10 months to review a standard ANDA under current rules. But many applications are incomplete or have issues, which delays approval. On average, it takes 18 to 24 months from first submission to final approval, especially if the company has to resubmit after a rejection.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same strict standards for quality, strength, purity, and stability as brand-name drugs. Studies show that 97% of generic drugs are therapeutically equivalent to their brand-name counterparts. There’s no evidence that generics are less safe.
Why do some generic drugs cost more than others?
When a new generic enters the market, the first company often gets a 180-day exclusivity period. During that time, they’re the only one selling it - so prices stay higher. Once other generics enter, competition drives prices down. Sometimes, if a drug has only one or two manufacturers, prices can stay high due to lack of competition.
10 Comments
Tiffany Adjei - Opong
January 6 2026
Oh please. You think the FDA is protecting us? They approve 700+ generics a year and barely read half of them. I worked at a contract lab that ran bioequivalence studies for generics - we’d tweak the formulation just enough to pass, then quietly change it after approval. The ‘same active ingredient’? Yeah, right. The excipients? Totally different. And the FDA doesn’t even test the final product unless someone dies. It’s a rigged game.
Isaac Jules
January 7 2026
Wow. Just wow. So you’re saying the 90% of prescriptions filled with generics are basically placebo pills? 🤡
Joann Absi
January 9 2026
AMERICA IS BEING POISONED BY INDIAN CHEMICAL FACTORIES. 🇮🇳🇨🇳 The FDA lets Chinese labs make 80% of our active ingredients. Do you know what’s in that ‘generic’ metformin? Maybe rat poison. Maybe illegal fentanyl analogs. They don’t test it because they’re too busy taking bribes. This isn’t healthcare - it’s a global scam. 🇺🇸🔥
Katelyn Slack
January 11 2026
i think the part about the 180 day exclusivity is super important but i always forget how it works. like why does the first one get to charge more? is that fair? 🤔
Melanie Clark
January 11 2026
It is a well documented fact that the FDA has been compromised by corporate interests and the pharmaceutical industry has systematically dismantled public health safeguards under the guise of cost reduction. The ANDA process is not a triumph of efficiency - it is a surrender to profit-driven negligence. Patients are being sacrificed on the altar of shareholder value. There is no oversight. No accountability. No justice. The system is broken. And you are complicit if you accept it.
Saylor Frye
January 12 2026
Look, I get it - generics save money. But let’s be real: most of these companies are just repackaging old chemistry. The real innovation died when Big Pharma stopped investing in new molecules and started gaming patent cliffs. ANDA isn’t progress. It’s pharmaceutical stagnation with a nice marketing label.
Kiran Plaha
January 12 2026
in india we make most of these generics. many people here dont even know what brand names are. we just take the formula and make it cheap. its good for the world. but yes, sometimes quality is bad. not all factories are clean. but its not like they are lying. its just hard to control everything.
Amy Le
January 14 2026
And yet, the same people who scream about ‘corporate greed’ in pharma are the ones who demand $4 insulin. 🤦♀️ You want cheap? Then accept that someone else did the hard work. The brand-name company spent $2B and 15 years. The generic company spent $2M and 2 years. You’re not getting a miracle - you’re getting a free ride.
Pavan Vora
January 15 2026
From India, I can say: we take pride in making these drugs. We have factories that meet USFDA standards - yes, really! But sometimes, the pressure to deliver cheaply leads to corners… not always cut, but sometimes… bent. Still, millions of lives are saved because of ANDA. Don’t forget that. 🙏





Wesley Pereira
January 5 2026
ANDA? More like A Nasty Delusion for Big Pharma’s bottom line. The FDA’s ‘bioequivalence’ standards are a joke - 80-125% range? That’s like saying your insulin is ‘close enough’ if it sometimes makes you pass out or sometimes does nothing. I’ve seen patients crash because the generic had a different filler that altered absorption. They call it science. I call it gambling with people’s lives.