
When you pick up a prescription for a generic drug, you might not think twice about whether it works the same as the brand-name version. But behind that simple swap is a detailed, science-backed system called Therapeutic Equivalence Codes-or TE Codes. These codes tell pharmacists exactly which generic drugs can be safely substituted for brand-name medicines without changing how well they work. It’s not just a label. It’s a legal and clinical standard that saves billions every year and keeps millions of patients on their medications without breaking the bank.
What Are TE Codes and Why Do They Matter?
Therapeutic Equivalence Codes are assigned by the U.S. Food and Drug Administration (FDA) and published in the Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. First created in 1984 after the Hatch-Waxman Act, TE Codes were designed to bring clarity to generic drug substitution. Before this system, pharmacists in different states had different rules about when they could swap a brand drug for a generic. Some couldn’t substitute at all. Others had to check with the doctor every time. TE Codes changed that.
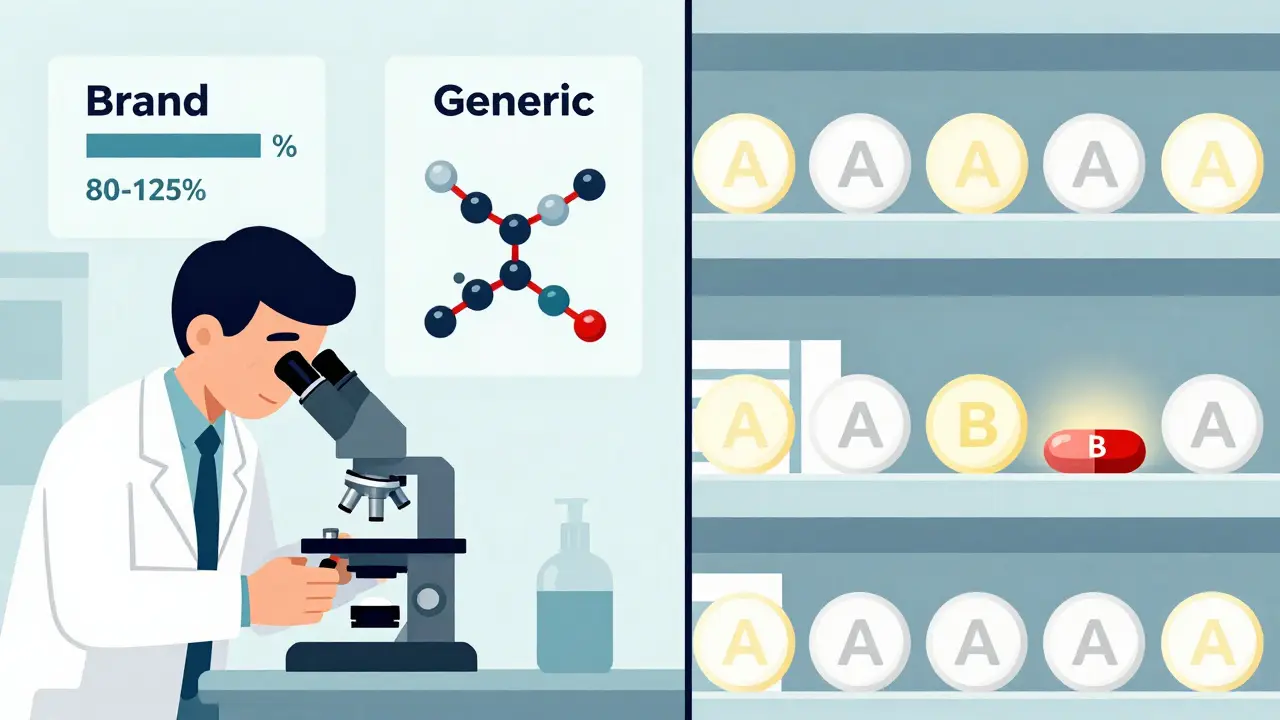
The code itself is simple: one or two letters. But each letter carries weight. If a drug has an “A” code, it means the FDA has reviewed it and found it to be therapeutically equivalent to the brand-name version. That’s the green light for substitution. If it’s a “B” code, the FDA says it’s not equivalent-either because the generic doesn’t absorb the same way in the body, or because it has different inactive ingredients that affect performance. In that case, the pharmacist must give you the brand unless the doctor says otherwise.
These codes are more than just guidelines. All 50 U.S. states use them as the legal basis for automatic generic substitution laws. That means if your prescription is for a drug with an “A” rating, your pharmacist can-and often will-give you the cheaper generic version without calling your doctor. This system is why over 90% of prescriptions filled in the U.S. are for generics today.
How the FDA Determines Therapeutic Equivalence
The FDA doesn’t just guess whether a generic works the same. It requires proof. For a drug to get an “A” rating, it must meet three strict criteria:
- Pharmaceutical equivalence: The generic must have the same active ingredient, strength, dosage form, and route of administration as the brand. If the brand is a 10mg tablet taken by mouth, the generic must be exactly the same.
- Bioequivalence: The generic must deliver the same amount of active ingredient into your bloodstream at the same rate. This is tested in clinical studies where volunteers take both the brand and generic versions. The FDA requires the generic’s absorption levels (measured as AUC and Cmax) to fall within 80-125% of the brand’s. That’s a tight window-and it’s why most generics pass.
- Clinical equivalence: The generic must produce the same therapeutic effect and safety profile under normal use conditions. This is usually inferred from the first two criteria, but for complex drugs, the FDA may require additional data.
For example, a generic version of atorvastatin (the active ingredient in Lipitor) with an “AA” code means it’s a powder for oral suspension and has been proven equivalent. The “AA” tells you the form, and the “A” tells you it’s safe to swap. The same goes for “AP” (powder for injection), “AN” (injectable solution), or “AT” (topical cream).
These evaluations are done through the Abbreviated New Drug Application (ANDA) process, which lets generic manufacturers skip expensive clinical trials by relying on the brand’s safety data-so long as they prove bioequivalence. It’s how generics can cost 80-85% less than brand-name drugs.
What TE Codes Don’t Tell You
Despite their rigor, TE Codes aren’t perfect. They’re designed for simple, well-understood drugs. But they struggle with complex products.
Take inhalers. Two generic budesonide inhalers might have identical active ingredients and pass bioequivalence tests-but if the spray pattern, particle size, or delivery mechanism is slightly different, the drug might not reach the lungs the same way. In 2019, the FDA withdrew TE ratings for several generic inhalers after reports of inconsistent performance. The same issue came up with topical steroids and some injectables.
Narrow therapeutic index (NTI) drugs are another gray area. These are medications where even tiny differences in blood levels can cause serious side effects or treatment failure. Warfarin (a blood thinner), levothyroxine (for thyroid disorders), and phenytoin (for seizures) fall into this category. While many of these drugs have “A” ratings, some patients report feeling different after switching-even when the FDA says they’re equivalent. A 2022 study found that 12.7% of patients perceived changes after switching between TE-rated generics. For some, it’s a real issue. For others, it’s psychological. But the FDA acknowledges the concern and is exploring ways to improve evaluations for these drugs.
Also, TE Codes only apply to multi-source drugs-those with more than one manufacturer. If a drug has only one generic version (a “single-source” generic), the FDA doesn’t assign a TE Code because there’s nothing to compare it to. That means no automatic substitution rules apply.
How Pharmacists Use TE Codes Every Day
For pharmacists, TE Codes are part of the routine. When a prescription comes in, they check the drug’s TE rating in the Orange Book-either through their pharmacy software, the FDA’s online database, or a mobile app. If it’s an “A” code and the prescriber hasn’t marked “dispense as written,” they substitute automatically.
It’s fast. Most pharmacy systems flag the TE code right on the screen. The average time to verify a TE code is less than 30 seconds, according to the Pharmacy Technician Certification Board. And it’s accurate: 91% of U.S. pharmacists say they have high confidence in TE-rated substitutions.
Pharmacy schools now teach TE Codes as core knowledge. Nearly all new pharmacists pass the NAPLEX exam with strong scores on TE Code interpretation. And pharmacy benefit managers (PBMs), hospitals, and insurers all rely on TE Codes to design formularies and control costs.
But it’s not flawless. Sometimes, state formularies lag behind the latest Orange Book updates. In 2022, about 3.2% of substitutions were affected by these delays. The FDA’s Orange Book Help Desk handles around 1,200 questions a month to clear up confusion. And while the system is robust, it’s not meant to replace clinical judgment. If a patient has had a bad reaction to a specific generic, the doctor can-and should-write “do not substitute.”
Why TE Codes Save Billions
The financial impact is massive. Since 1995, TE Codes have helped save over $2.2 trillion in U.S. healthcare spending, according to former FDA Director Dr. Janet Woodcock. In 2022 alone, generic drugs saved patients and insurers an estimated $130 billion. That’s because a brand-name drug might cost $300 a month, while its TE-rated generic costs $20.
That price difference changes behavior. A patient who can’t afford Lipitor might skip doses-or stop taking it altogether. But with a $20 generic, they’re far more likely to stick with the treatment. That’s why TE Codes aren’t just about cost-they’re about access and outcomes.
Market data shows the effect: within six months of a generic entering the market with an “A” rating, the brand’s share drops from over 40% to under 25%. For non-TE-rated generics, the brand keeps nearly half its market. That’s the power of confidence.

What’s Next for TE Codes?
The FDA is working to expand the system. In 2022, it released new guidance to standardize how TE evaluations are done, especially for complex drugs. A pilot program is testing whether real-world patient data-like electronic health records and pharmacy claims-can help improve equivalence assessments.
Biosimilars (the biologic version of generics) are next. The FDA plans to introduce new TE-like codes for them by 2024. Right now, biosimilars are evaluated under different rules, but the goal is to create a similar framework so pharmacists can substitute them with confidence.
Industry experts predict that by 2027, over 93% of all prescriptions in the U.S. will be for TE-rated generics. That’s up from 90.1% today. But the real challenge isn’t technical-it’s perception. Some patients still believe generics are “weaker” or “inferior.” The data says otherwise. But education matters. That’s why the FDA runs monthly webinars and partners with pharmacy associations to spread the word.
What You Should Know as a Patient
If you’re on a chronic medication-like high blood pressure, diabetes, or thyroid medicine-chances are you’ve been switched to a generic. That’s normal. And if it’s a TE-rated product, you can trust it. The FDA doesn’t approve generics lightly. The science is solid.
But if you notice a change in how you feel after a switch-dizziness, fatigue, unusual side effects-don’t ignore it. Talk to your doctor or pharmacist. It could be a formulation difference, a psychological effect, or something unrelated. Either way, your feedback helps improve the system.
And if your doctor writes “dispense as written” on your prescription, that’s their choice. Maybe they’ve seen you respond better to one brand. Maybe they’re cautious with NTI drugs. Respect that. But if you’re paying more for a brand name and a generic is available with an “A” code, ask your pharmacist: “Is there a TE-rated generic I could use instead?” You might save hundreds a year.
TE Codes are one of the most successful public health tools in modern medicine. They balance safety, science, and savings. They’re not perfect, but they work-better than almost any other system in the world.
What does an 'A' rating mean in TE Codes?
An 'A' rating means the FDA has determined the generic drug is therapeutically equivalent to the brand-name drug. It has the same active ingredient, strength, dosage form, and route of administration, and it performs the same way in the body. Pharmacists can legally substitute it without needing approval from the prescriber.
Can I request a brand-name drug even if a generic is available?
Yes. Even if a generic has an 'A' rating, you can ask your pharmacist to give you the brand-name version. You may have to pay more out of pocket, especially if your insurance doesn’t cover the brand. Some people prefer brands due to past experiences, but for most drugs, the generic works just as well.
Why do some people feel different after switching to a generic?
Some patients report changes in how they feel after switching, even when the generic is TE-rated. This can happen with narrow therapeutic index drugs like warfarin or levothyroxine, where small differences in absorption may matter. It can also be psychological-people expect generics to be less effective. In most cases, clinical tests show no difference. But if you notice real symptoms, talk to your doctor.
Are all generic drugs assigned a TE Code?
No. Only multi-source drugs-that is, those with more than one manufacturer-receive TE Codes. If a drug has only one generic version, the FDA doesn’t assign a code because there’s nothing to compare it to. Also, single-source drugs, biologics, and some complex products like inhalers may not have TE ratings even if generics exist.
How do I check a drug’s TE Code?
You can look up any drug’s TE Code in the FDA’s online Orange Book database at fda.gov/orangebook. Most pharmacy systems also display the code automatically when filling a prescription. If you’re unsure, ask your pharmacist-they’re trained to use it and can explain what the code means for your medication.
11 Comments
Swapneel Mehta
December 22 2025
It’s fascinating how such a simple two-letter code can impact global access to medicine. In India, generics are the only option for most people. Knowing there’s a system like this that ensures safety and consistency gives me hope. The Orange Book should be translated into more languages.
Teya Derksen Friesen
December 24 2025
It is imperative to recognize that the Therapeutic Equivalence Code system represents one of the most meticulously designed public health frameworks in modern pharmacology. The FDA’s adherence to bioequivalence thresholds, coupled with state-level legal codification, ensures that cost containment does not come at the expense of therapeutic integrity. This is a model for other nations.
Sandy Crux
December 25 2025
Let’s be honest: the FDA’s ‘A’ rating is a political compromise. They don’t test for long-term outcomes. They don’t account for individual variability in CYP450 metabolism. And they ignore the fact that inactive ingredients-like dyes, lactose, or preservatives-can trigger reactions in sensitive patients. You think you’re getting the same drug? You’re not. You’re getting a legally approved approximation. And that’s terrifying.
Hannah Taylor
December 25 2025
bro the FDA is totally in bed with big pharma. they only give A ratings to generics made by companies that donated to their PACs. i read this one reddit thread where a guy said his generic adderall made him feel like a zombie-turns out the filler was cornstarch from a banned farm. they’re hiding this. #orangebooklies
Ben Warren
December 26 2025
It is both scientifically and ethically indefensible to suggest that therapeutic equivalence can be reliably established through bioequivalence studies conducted on a cohort of 24 healthy adult males under fasting conditions. The population that actually uses these medications includes geriatric patients with polypharmacy, patients with renal or hepatic impairment, and those with comorbid psychiatric conditions-all of whom are systematically excluded from the bioequivalence trials upon which TE ratings are predicated. To assert that a 125% Cmax variance is clinically insignificant is not merely inaccurate-it is a dangerous oversimplification that prioritizes fiscal efficiency over patient safety. The FDA’s reliance on the ANDA pathway, while economically expedient, constitutes a systemic abdication of its duty to ensure therapeutic fidelity across heterogeneous patient populations.
Peggy Adams
December 28 2025
generic drugs are just brand names with a different label. i switched to a generic blood pressure med and my head felt like a balloon. my pharmacist said ‘it’s the same thing’-yeah right. i bet they use chinese chemicals and don’t even test it. why do you think the brand name lasts longer? because it works.
Sarah Williams
December 28 2025
My grandma’s on levothyroxine. She switched generics twice and felt fine both times. But when she got a different batch last month, she got dizzy. She called her pharmacist-they checked the TE code, called the manufacturer, and swapped it out within an hour. That’s the system working. Don’t panic. But do speak up if something feels off.
Theo Newbold
December 29 2025
TE codes are a facade. The bioequivalence window of 80-125% is absurd. That’s a 45% variance. If two drugs can differ by that much and still be called ‘equivalent,’ then the term is meaningless. And don’t get me started on how the FDA ignores real-world data until someone dies. This isn’t science-it’s regulatory theater.
Jay lawch
December 29 2025
India produces 60% of the world’s generics, yet the FDA still insists on its own flawed standards. The U.S. refuses to accept Indian-manufactured generics with identical bioequivalence data because of outdated GMP biases. This isn’t about safety-it’s about protectionism. The Orange Book is a tool of American economic imperialism disguised as public health. We have better manufacturing standards than most U.S. facilities. The FDA should be auditing its own suppliers, not bullying global producers.
Christina Weber
December 30 2025
The notion that a 125% Cmax variance is ‘within acceptable limits’ is statistically indefensible and clinically irresponsible. The FDA’s reliance on point estimates rather than confidence intervals, coupled with its failure to mandate post-marketing pharmacovigilance for TE-rated generics, constitutes a fundamental breach of the precautionary principle. Moreover, the absence of standardized labeling for excipients-despite documented hypersensitivity reactions-represents a regulatory blind spot of alarming magnitude. Patients deserve transparency, not bureaucratic euphemisms.




Jon Paramore
December 22 2025
An 'A' rating means pharmaceutical and bioequivalence are met under FDA guidelines-same API, same PK profile, same route. The 80-125% AUC/Cmax window is tight, and it’s not arbitrary. It’s based on decades of pharmacokinetic modeling. If you’re on a narrow TI drug like warfarin, yeah, monitor INR after switch. But for 95% of prescriptions? No difference. The data is overwhelming.