Tag: RSABE
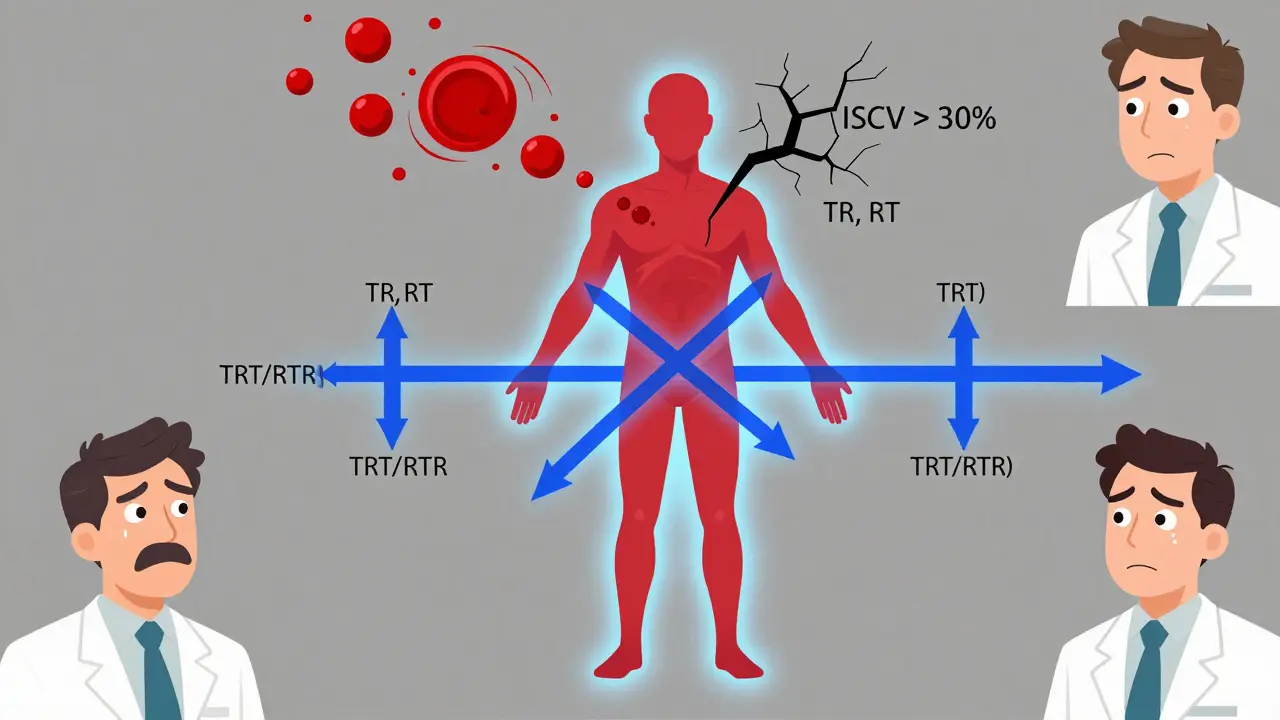
Replicate Study Designs: Advanced Methods for Bioequivalence Assessment
Replicate study designs are essential for assessing bioequivalence of highly variable drugs. They reduce sample sizes, improve success rates, and enable regulatory acceptance through reference-scaling. Learn which design to use, how to avoid common pitfalls, and what the industry is doing in 2026.
View MorePopular Posts

Post Categories
Tag Clouds
- online pharmacy
- medication safety
- drug interactions
- medication adherence
- alternatives
- COPD treatment
- side effects
- online pharmacy UK
- generic drugs
- biosimilars
- Sildenafil
- antidepressants
- online pharmacies
- dietary supplements
- blood pressure
- talk to doctor
- beta-blockers
- antibiotics
- antibiotic resistance
- serotonin




