FDA Drug Recalls: What You Need to Know About Unsafe Medications
When the FDA drug recalls, official actions by the U.S. Food and Drug Administration to remove unsafe or mislabeled medications from the market. Also known as pharmaceutical withdrawals, these recalls are triggered when a drug is found to contain harmful contaminants, incorrect dosages, or hidden side effects that weren’t disclosed during approval. It’s not just about a bad batch—it’s about real people getting sick or worse because a medication they trusted turned out to be dangerous.
FDA drug recalls often involve contaminated generics, cheaper versions of brand-name drugs that fail quality control, like the NDMA-laced ranitidine or the tainted valsartan that flooded pharmacies in 2018. These aren’t rare events. The FDA issues over 1,000 recalls a year, and many affect drugs people take daily—blood pressure pills, antidepressants, even thyroid meds. One of the most common causes? Poor manufacturing practices overseas, where oversight is weaker and cost-cutting is routine. Another? Drug interactions that weren’t caught until thousands of patients started bleeding, having heart issues, or developing liver damage. The drug interaction, when two or more medications react in the body to create unexpected, often dangerous effects is a silent killer, and recalls sometimes follow after reports pile up.
You might think recalls mean your pill is unsafe right now, but that’s not always true. Some recalls are limited to specific lots, batch numbers, or expiration dates. Others target entire classes of drugs—like calcium channel blockers contaminated with nitrosamines, or SSRIs linked to increased bleeding risk. The medication safety, the practice of ensuring drugs are used correctly and without harm starts with knowing what’s been pulled. If you’re on a drug that was recently recalled, your pharmacist can tell you if your bottle is affected. You don’t need to panic, but you do need to act. Check the FDA’s recall page, look up your lot number, and talk to your doctor before stopping anything cold. Many people don’t realize that switching meds without guidance can be just as risky as staying on a recalled drug.
The posts below dig into real cases where drugs caused harm—not because they were designed to, but because of hidden flaws, bad labeling, or dangerous combos. You’ll find deep dives on how grapefruit messes with blood pressure meds, why switching thyroid generics needs monitoring, and how common painkillers can trigger heart problems. These aren’t theoretical warnings. They’re based on actual recalls, patient reports, and FDA alerts. What you learn here could keep you—or someone you care about—out of the hospital.
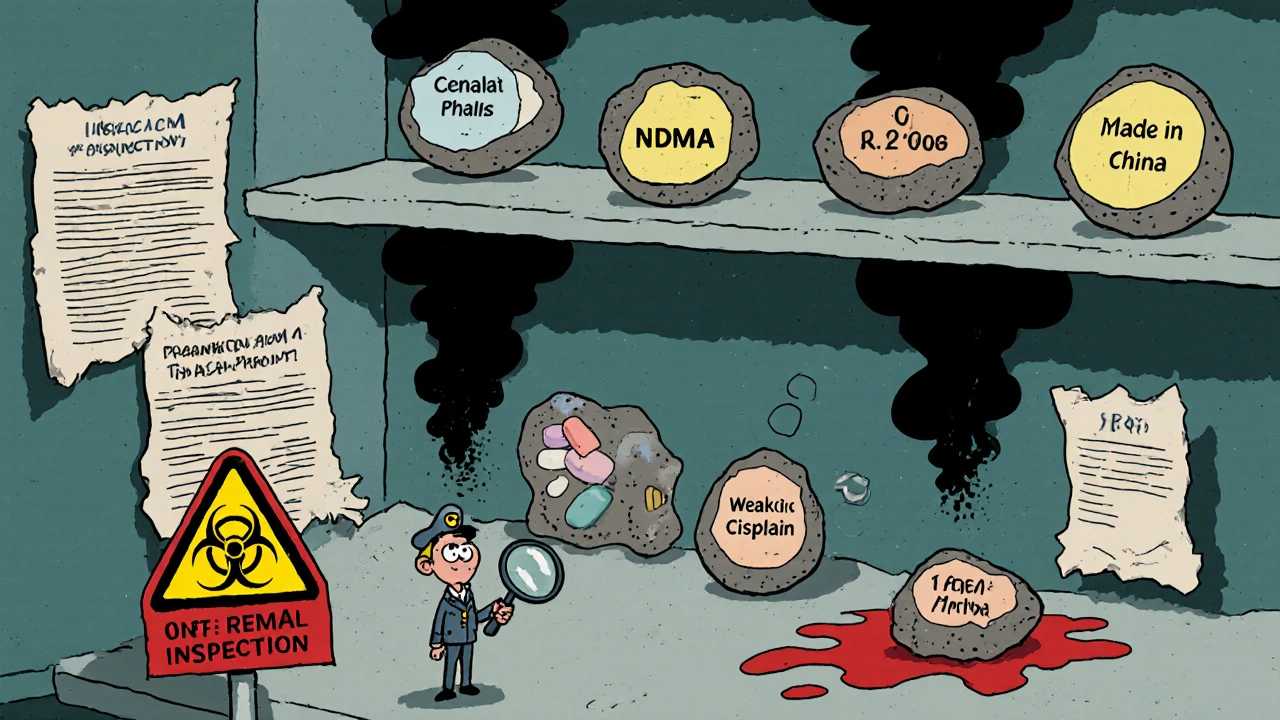
Contamination Issues in Generic Drugs: Recent Cases and How to Prevent Them
Recent cases show dangerous contaminants like NDMA and benzene in generic drugs, raising serious safety concerns. Learn what’s being recalled, who’s at risk, and how to protect yourself.
View More