
When you order medication online, you’re trusting a website with your health. But not all online pharmacies are legitimate. Some sell fake pills, expired drugs, or no medicine at all. In 2025, the FDA issued 147 warning letters to illegal online pharmacies - a 32% jump from 2024. So who’s actually watching these sites? The answer isn’t just one agency. It’s a system: the FDA at the federal level, and 50 different state boards of pharmacy working together - sometimes in sync, sometimes at odds.
What the FDA Actually Does
The FDA doesn’t license online pharmacies. That’s a common mistake. Instead, it watches what’s being sold. If a website sells unapproved drugs - like counterfeit versions of Ozempic or Mounjaro - the FDA steps in. Their main job is safety: making sure drugs are what they say they are, contain the right dose, and come with proper warnings.
They also crack down on false advertising. In 2025, the FDA and HHS announced new rules targeting direct-to-consumer ads on social media. Influencers promoting GLP-1 weight-loss drugs without mentioning side effects like nausea or pancreatitis? That’s now a target. The FDA’s Office of Prescription Drug Promotion issued zero warning letters in 2024 - but that’s not because things got better. It’s because they shifted focus. Now they’re going after digital ads, not just print.
The BeSafeRx tool on the FDA’s website lets you check if a pharmacy is real. Type in the site’s name, and it tells you if the pharmacy is licensed by a state board. If it’s not listed? Don’t buy from it. In Q3 2025, over 1.2 million people used BeSafeRx - up 40% from the year before. People are learning to check before they click.
State Boards: The Real Gatekeepers
Here’s the truth: the FDA can’t shut down a website without help from the states. That’s because state pharmacy boards issue the actual licenses. Every legitimate online pharmacy must be licensed in the state where it operates. Forty-eight out of fifty states have public databases where you can search for licensed pharmacies. California, Texas, and Florida reported the most complaints in 2024 - not because they have more bad pharmacies, but because they have more people using them.
State boards also handle complaints. If you get a pill that looks wrong, or your prescription never arrives, you report it to your state board. They can fine the pharmacy, suspend its license, or even refer the case to the DEA for criminal charges. In August 2025, QuickMedsOnline.com was hit with a $500,000 penalty for repeatedly selling prescriptions without valid doctor orders.
But here’s the problem: rules vary by state. Twenty-seven states have extra rules on top of federal law. Some require in-person exams before telemedicine prescriptions. Others limit how many controlled substances a provider can prescribe remotely. That’s why a pharmacy licensed in Florida might be illegal in New York - even if both are FDA-compliant.
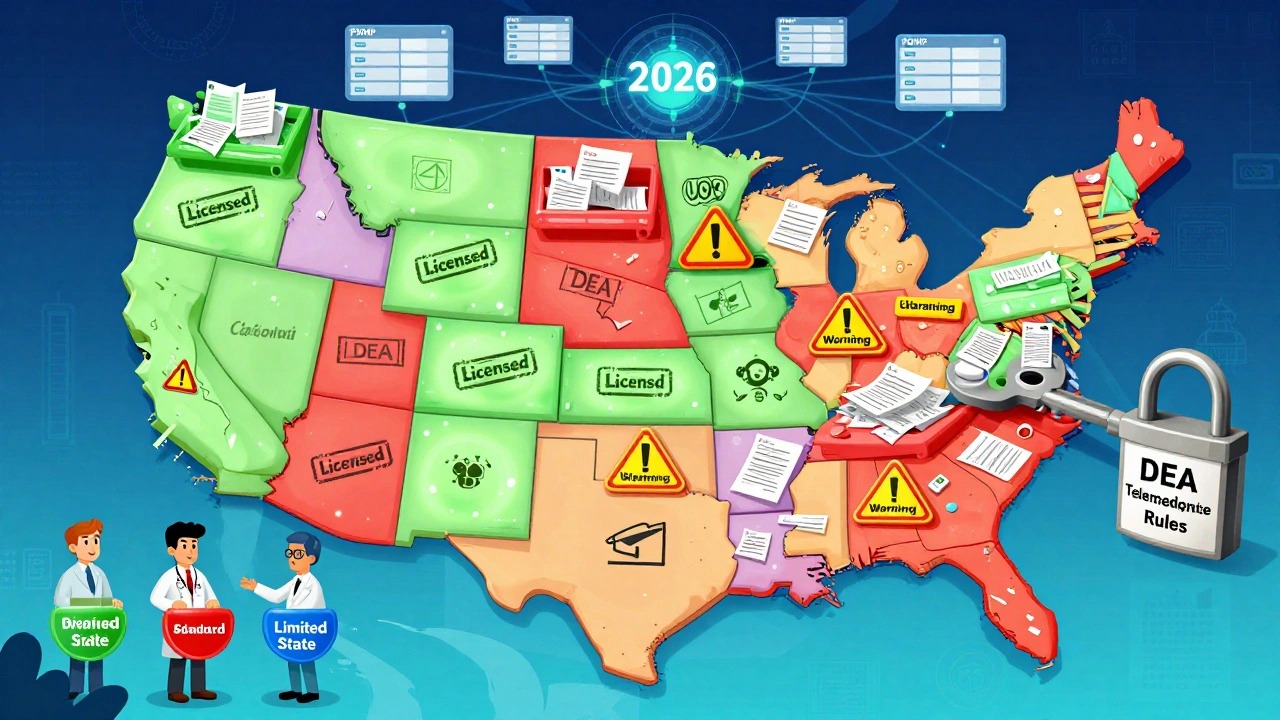
DEA Rules: Controlled Substances and Telemedicine
If you’re ordering opioids, stimulants, or sedatives online, the DEA is involved. Before 2025, the Ryan Haight Act required an in-person visit before any controlled substance could be prescribed remotely. That rule was relaxed during COVID, and then made permanent - but only for certain providers.
In January 2025, the DEA rolled out three new Special Registrations:
- Standard Registration: Lets providers prescribe Schedule III-V drugs (like Xanax or tramadol) via telemedicine - no in-person visit needed, but they must check the state’s Prescription Drug Monitoring Program (PDMP) first.
- Advanced Registration: Only for psychiatrists, hospice doctors, pediatricians, and long-term care providers. Allows them to prescribe Schedule II drugs (like Adderall or oxycodone) remotely - if they meet strict training and patient review rules.
- Limited State Registration: For providers in states that still require in-person visits. Lets them operate under state rules while still being DEA-registered.
These changes mean more patients can get needed medications without driving hours to a clinic. But they also mean more oversight. The DEA is building a nationwide PDMP - something that didn’t exist before. Right now, providers have to check 50 different state systems. By 2026, they’ll have one central database.
Compounding Pharmacies: The Gray Zone
When drugs like semaglutide and tirzepatide were in short supply in 2024, compounding pharmacies stepped in. These are pharmacies that mix custom doses - not mass-produced pills. The FDA doesn’t approve these drugs before they’re sold. That’s the key point: compounded drugs aren’t FDA-reviewed.
Only 503A pharmacies - the kind that make drugs for individual patients with a valid prescription - can legally compound these medications. 503B pharmacies, which make bulk batches for clinics, can’t touch them unless they’re on the FDA’s shortage list. And as of September 2025, those drugs were removed from the list - meaning 503B pharmacies can no longer make them at scale.
That left the market to 503A pharmacies. But because they’re regulated by states, not the FDA, oversight is patchy. One state might require strict quality controls. Another might not. That’s why the FDA warns: if a website offers “custom” versions of brand-name drugs at half the price, it’s likely not following the rules.

What to Look For - And What to Avoid
Here’s how to tell if an online pharmacy is safe:
- It requires a valid prescription - no exceptions.
- It has a U.S. physical address and phone number you can call.
- A licensed pharmacist is available to answer questions.
- It’s listed on the FDA’s BeSafeRx site or has VIPPS accreditation (from the National Association of Boards of Pharmacy).
- It doesn’t offer “miracle cures” or “no-exam” prescriptions for controlled substances.
Red flags:
- Prices way lower than CVS or Walgreens - if it’s too good to be true, it is.
- No contact info, or only a PO box.
- Offers to sell drugs without a prescription.
- Sells drugs not approved in the U.S. - like versions of insulin from Mexico or India.
- Asks for payment only in cryptocurrency or wire transfer.
Real users report bad experiences: pills that don’t work, side effects no one warned them about, or no medicine at all. On Reddit’s r/Telehealth, users in September 2025 shared 87 stories of receiving counterfeit meds. Meanwhile, verified pharmacies like CVS Caremark Online have a 4.6/5 rating from over 12,000 reviews.
The Bigger Picture: Why This System Exists
There’s no perfect system. The FDA can’t police every website. State boards are underfunded and overwhelmed. The DEA’s new rules help, but they’re complex. And patients? They just want affordable, fast access to their meds.
The goal isn’t to stop online pharmacies. It’s to stop the bad ones. In 2025, 37% of U.S. adults used an online pharmacy - up from 22% in 2020. But 78% of those users stuck with pharmacies tied to brick-and-mortar chains like CVS, Walgreens, or Kroger. Those are the ones with the strongest compliance records.
The future? A more connected system. The nationwide PDMP will help. Real-time verification of telemedicine scripts by the end of 2026 will help. And more enforcement - especially on social media - will make it harder for bad actors to hide.
But your best protection? Stay informed. Check the pharmacy. Don’t skip the prescription. And if something feels off - report it. Your state board is waiting to hear from you.
10 Comments
Karl Barrett
December 6 2025
The structural fragmentation of pharmaceutical regulation is a textbook case of regulatory arbitrage. The FDA’s jurisdictional limitations under the FDCA, combined with the 10th Amendment-anchored sovereignty of state pharmacy boards, create a patchwork enforcement regime where compliance is probabilistic, not absolute. The DEA’s new special registrations attempt to harmonize telemedicine prescribing under controlled substance statutes, yet the absence of a unified PDMP infrastructure until 2026 leaves a dangerous latency window for diversion. Compounding pharmacies under 503A operate in a regulatory gray zone precisely because they’re exempt from premarket approval-this isn’t oversight, it’s delegation by default.
Jake Deeds
December 7 2025
People just don’t want to work for their meds anymore. You want cheap Ozempic? Go see a doctor. Pay for a visit. Wait your turn. But no, let’s just click a button and hope some guy in a basement in India sends you a pill that might as well be a placebo. And now the FDA has to babysit everyone’s poor life choices. I mean, really. If you’re too lazy to drive 20 minutes to CVS, maybe you shouldn’t be on a weight-loss drug in the first place.
val kendra
December 8 2025
BeSafeRx is your best friend. Use it. Every time. Even if the site looks clean. Even if the price is half. Even if they say ‘FDA approved’-they’re lying. I checked a site that looked like Walgreens’ cousin. BeSafeRx said ‘unlicensed.’ I reported it. Two weeks later, the site vanished. State boards do work. They’re just buried under 10,000 complaints. Don’t wait for them to find you. Find them first.
Isabelle Bujold
December 10 2025
It’s interesting how the Canadian model handles this-Health Canada works in tandem with provincial pharmacy colleges, and there’s a single national portal for verifying online pharmacies. We don’t have the same patchwork chaos because we centralized our verification system back in 2018. The U.S. could do this too, but there’s too much political inertia around state rights. I get the federalism argument, but when people are getting fake insulin, federalism doesn’t save lives. A single, federally mandated verification portal with real-time API integration to state boards would cut fraud by 80% in under a year. It’s technically feasible. It’s just not politically sexy.
Ollie Newland
December 11 2025
DEA’s new special registrations are a step forward, but the real issue is the PDMP fragmentation. I’ve had to check six different state systems just to prescribe tramadol to a patient who moved from Ohio to Georgia. By 2026, if they finally roll out that national system, it’ll be a godsend. But until then? We’re all playing whack-a-mole with prescription fraud. And yeah, social media ads are a nightmare. I saw one yesterday-‘Lose 20lbs in 2 weeks with this miracle shot!’ No side effects mentioned. Just a guy in a tank top holding a vial. That’s not marketing. That’s criminal.
Rebecca Braatz
December 11 2025
If you’re using an online pharmacy, you owe it to yourself to check the license. It takes 30 seconds. Use BeSafeRx. Look up the phone number. Call them. Ask if a pharmacist is on staff. If they hesitate? Walk away. You’re not being paranoid-you’re being smart. And if you’ve been burned? Tell your state board. Don’t just vent on Reddit. File a complaint. That’s how they track patterns. That’s how they shut these places down. Your voice matters. Don’t stay silent.
Carolyn Ford
December 12 2025
You people are so naive. The FDA doesn’t protect you. They protect Big Pharma. They let compounding pharmacies sell semaglutide because they can’t make enough? That’s not oversight-that’s collusion. And don’t even get me started on VIPPS. That’s just a logo you pay for. I’ve seen VIPPS sites selling counterfeit Adderall. The whole system is rigged. You think a state board gives a damn? They’re underfunded, overworked, and probably on the payroll of some pharmacy conglomerate. Wake up.
Heidi Thomas
December 13 2025
Stop acting like this is new. People have been getting scammed online since 2005. The FDA doesn’t regulate websites. That’s not their job. Their job is to regulate drugs, not e-commerce. If you want to buy pills off a website, you’re the problem. Not the FDA. Not the state boards. YOU. Stop blaming systems for your bad decisions.
Alex Piddington
December 14 2025
Thank you for this comprehensive breakdown. As a healthcare provider, I’ve seen firsthand how patients fall for fraudulent pharmacies. The emotional appeal of ‘affordable meds’ often overrides rational caution. I’ve started including a printed checklist in every telehealth visit-BeSafeRx link, red flags, and how to report. Small step, but it’s working. Let’s keep pushing for public education, not just enforcement. Knowledge is the most effective filter.





Yasmine Hajar
December 5 2025
I ordered insulin from a site that looked legit-turned out it was just sugar pills. My glucose spiked so bad I ended up in the ER. FDA? They sent a warning letter. No one called me. No one fixed it. State boards? They said it was out of their jurisdiction. Guess what? I’m not trusting another website ever again.