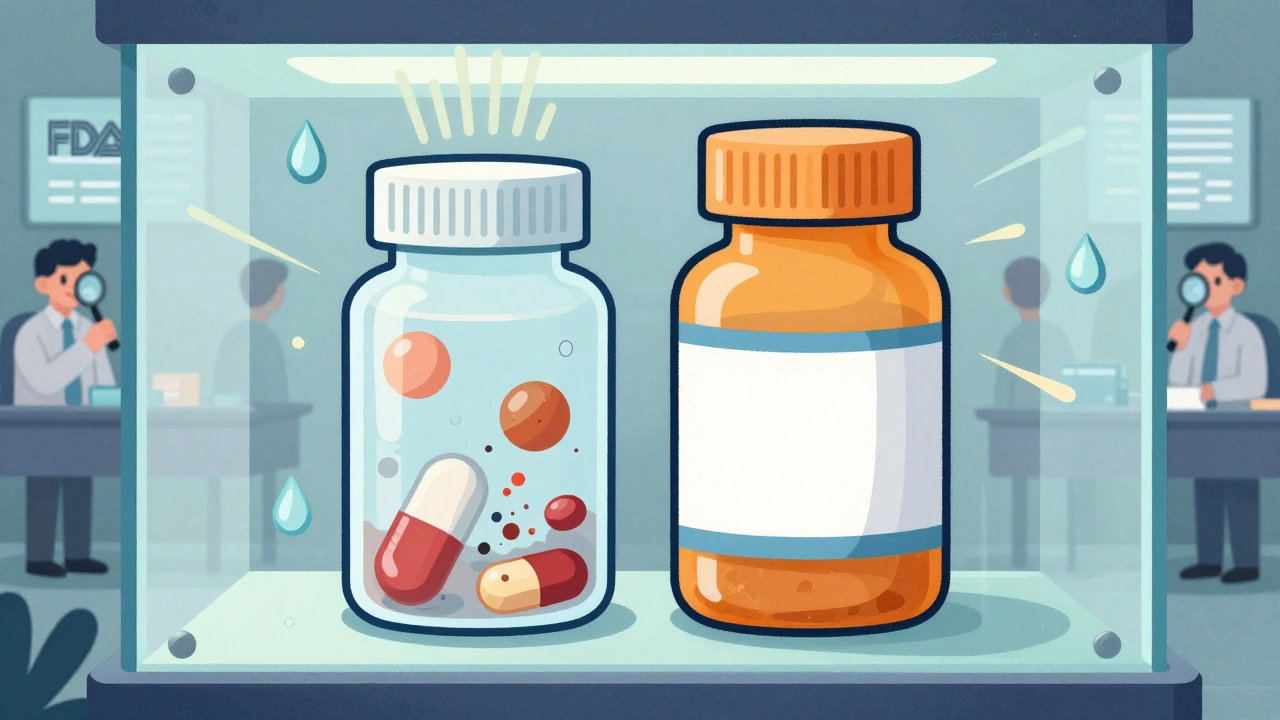
When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how does the FDA make sure that generic drugs don’t lose strength, break down, or become unsafe over time? The answer lies in stability testing-a rigorous, science-driven process that every generic drug must pass before it hits the market.
Why Stability Testing Matters for Generic Drugs
Generic drugs aren’t just copies. They’re required to be identical in active ingredient, strength, dosage form, and performance. But what happens after the bottle is sealed? Heat, humidity, light, and time can change a drug’s chemistry. If a tablet degrades, it might not work. Worse, it could create harmful impurities. That’s why the FDA demands proof that the drug stays safe and effective from the factory to the medicine cabinet.Stability testing isn’t optional. It’s the foundation of every Abbreviated New Drug Application (ANDA). Without it, the FDA won’t approve the product. And it’s not just about matching the brand-name drug-it’s about proving your specific version holds up under real-world conditions.
What the FDA Requires: The Core Rules
The FDA follows international standards set by the International Council for Harmonisation (ICH), especially ICH Q1A(R2). For generics, this means three non-negotiable requirements:- Test at least three primary batches of the drug product
- Each batch must be made at pilot scale under current Good Manufacturing Practices (cGMP)
- Test physical, chemical, biological, and microbiological properties that could change over time
These aren’t vague suggestions. They’re strict. The FDA wants data on things like tablet hardness, dissolution rate, moisture content, impurity levels, and microbial contamination. Even the packaging matters. The container and closure system used in testing must be exactly what you plan to sell-no shortcuts.
How Long Do You Need to Test?
There are two types of stability studies: long-term and accelerated.Long-term testing simulates real storage. For most drugs, that means keeping samples at 25°C ± 2°C and 60% ± 5% relative humidity. You need at least 12 months of data before you can submit your ANDA. But the FDA expects you to keep testing for the full shelf life you’re claiming-often 24 or 36 months.
Accelerated testing pushes the drug to its limits. Samples go into a chamber at 40°C ± 2°C and 75% ± 5% humidity. You need six months of data here. This helps predict how the drug will behave over time. If the drug shows major degradation in accelerated conditions, it’s a red flag.
Testing frequency matters too. For products with a shelf life of 12 months or more:
- First year: test every 3 months
- Second year: test every 6 months
- Each year after that: test annually
Missing a single time point can delay approval by months. The FDA doesn’t accept incomplete data.
What If You’re Making Multiple Strengths or Sizes?
You don’t always need to test every single version. The FDA allows two smart approaches: bracketing and matrixing.Bracketing means you test only the strongest and weakest strengths in a family, assuming the middle ones behave the same. For example, if you’re making 10mg, 20mg, and 40mg tablets, you might only test the 10mg and 40mg versions.
Matrixing means you test different batches at different times. Instead of testing every batch every month, you test a subset, rotating which ones are checked. This saves time and resources-but only if you can prove it’s scientifically valid. The FDA approves about two-thirds of these requests, but they’re picky about the reasoning.
Common Reasons Generic Drugs Get Rejected
Even with clear rules, many ANDAs fail. The FDA’s 2021 inspection report showed that 92.7% of stability-related deficiencies in generic applications came from just a few problems:- Missing or vague protocols-You need a detailed plan for every test, referencing USP chapters like <1151> and <1010>. If your protocol says “test for impurities,” that’s not enough. You must say which impurities, which method, which limits.
- Inadequate sampling-Too few samples, wrong timing, or poor handling. One study found 22.7% of failures came from sampling errors.
- Unvalidated test methods-If your lab can’t prove their test actually detects degradation, the data is useless. This was cited in over 30% of Complete Response Letters.
- Storage failures-Stability chambers must stay within ±2°C. FDA inspections found 63.2% of generic manufacturers had temperature excursions above that limit.
These aren’t minor oversights. They’re red flags that suggest poor quality control. And the FDA notices.
How Generics Differ from Brand-Name Drugs
You might think generics have it easier because they copy an existing drug. But that’s misleading.Brand-name companies spend years building stability data from scratch. Generics don’t have to do that. They can reference the reference listed drug’s (RLD) data to support their shelf-life claim. But here’s the catch: they still must test their own product. Why? Because even tiny changes in formulation, manufacturing, or packaging can affect stability.
For example, a generic manufacturer might use a different binder or coating. That could change how moisture enters the tablet. The brand’s data doesn’t help here. You have to prove your version is stable.
Another difference: generics rarely need forced degradation studies (where you stress the drug with heat, acid, or light). The brand’s data already shows how the active ingredient breaks down. Generics just need to show their version degrades the same way.

Real-World Challenges Manufacturers Face
Running stability studies isn’t cheap. According to a 2023 Tufts analysis, stability testing adds about $487,500 to the cost of each ANDA-roughly 18.7% of total development expenses.Smaller manufacturers, especially those outside the U.S., struggle with infrastructure. A 2022 FDA inspection found that 18.4% of stability data invalidations came from temperature deviations in storage chambers. Many facilities still use manual logbooks instead of automated monitoring systems. But the top 25 generic manufacturers? 78.4% now use digital systems that alert staff if conditions drift.
Training is another hurdle. It takes 6 to 9 months for staff to become proficient in ICH Q1A(R2) protocols. And if you’re new to the game, the learning curve can delay approval by over a year.
What’s Changing in 2025 and Beyond
The FDA isn’t standing still. In June 2025, they released a draft guidance proposing major updates:- 24 months of real-time stability data required for all new ANDAs (up from 12)
- Integration of Quality by Design (QbD) principles-meaning you must prove your process controls stability from day one
- New rules for drugs containing nanomaterials, which behave unpredictably over time
- Updated photostability requirements under ICH Q1C(R2), expected late 2025
Also, the FDA is piloting blockchain technology to verify stability data. Imagine a tamper-proof digital ledger that tracks every test result, storage condition, and sample change. If it works, it could cut down on fraud and errors.
Industry experts predict stability testing costs will rise 22.4% by 2027. But approval timelines might shrink-from 18.7 months to 14.2-because the rules are becoming clearer.
What This Means for Patients
You might wonder: why all this fuss? Can’t we just trust generics?The answer is yes-but only because of these tests. The FDA doesn’t approve a generic drug because it’s cheaper. They approve it because it’s proven to be as safe and effective as the brand. Stability data is what makes that possible.
Without it, a 6-month-old bottle of generic blood pressure medicine could lose 15% of its potency. That’s not theoretical. It’s happened. Stability testing prevents that.
And while some argue the rules are too strict-especially for well-established drugs-the FDA’s priority is patient safety, not cost savings. Every pill you take should work the same way, no matter when it was made or where it came from.
Do generic drugs need the same stability testing as brand-name drugs?
Yes, in practice. While brand-name companies build stability data from scratch, generics must still test their own product batches under the same ICH Q1A(R2) conditions. They can reference the brand’s data for context, but they can’t rely on it alone. The FDA requires direct evidence that their specific formulation, packaging, and manufacturing process maintain quality over time.
How long does stability testing take before a generic drug is approved?
You need at least 12 months of real-time data and 6 months of accelerated data to submit an ANDA. But the FDA expects you to continue testing beyond submission. Approval often happens before the full shelf life is reached, as long as the data shows the drug will remain stable through its proposed expiration date. Most applications are approved with 12-18 months of real-time data, assuming the accelerated data supports the claim.
Can a generic drug use the same expiration date as the brand-name drug?
Only if the generic manufacturer’s own stability data supports it. The brand’s expiration date isn’t automatically transferable. Even if the active ingredient and dosage are identical, differences in excipients, manufacturing, or packaging can change how quickly the drug degrades. The FDA requires proof-your own data-that your product lasts just as long.
What happens if a generic drug fails stability testing?
The FDA issues a Complete Response Letter, which means the application is not approved. The manufacturer must fix the issue-usually by improving the formulation, changing packaging, or running more tests-and resubmit. Stability issues are the #1 reason for rejection in generic drug applications, accounting for over 34% of all deficiency letters in 2019.
Are there exceptions to stability testing for older generic drugs?
Not currently. Even if a drug has been on the market for decades, any new manufacturer wanting to produce it must still run full stability studies. However, the FDA does allow bracketing and matrixing to reduce the number of tests. Some proposed legislation, like the 2024 Enhancing Generic Drug Access Act, suggests reducing requirements for well-established generics, but no changes have been finalized as of 2025.
Why do Indian manufacturers have more stability-related rejections?
In 2022, Indian manufacturers accounted for 40.3% of U.S. generic approvals but 62.8% of stability-related Complete Response Letters. This isn’t because their products are inferior-it’s often due to resource gaps. Many facilities lack automated environmental monitoring, trained staff, or robust quality systems. The FDA’s increased scrutiny under GDUFA has made these weaknesses more visible. But many Indian companies are now investing heavily in compliance to meet global standards.
12 Comments
Tommy Watson
December 13 2025
bruh why do we even bother?? like i just want my blood pressure pill to not kill me, not read a 50 page i ch q1a(r2) doc. they test the same drug 17 times just bc one company used a different binder?? smh. #genericdrugstruggle
Donna Hammond
December 15 2025
Actually, this is incredibly important. Stability testing isn’t red tape-it’s the only thing keeping you from taking a tablet that’s chemically degraded into something toxic. Moisture ingress, oxidation, photodegradation-these aren’t theoretical risks. I’ve seen data where expired generics lost up to 22% potency. That’s not a placebo effect. That’s a medical failure waiting to happen. The FDA’s standards are strict because lives depend on it.
Willie Onst
December 16 2025
Man, I love how we treat medicine like it’s a sci-fi lab experiment. You take a pill, it’s supposed to work-like a toaster toasts bread. But now we need climate-controlled chambers, blockchain logs, and PhDs to explain why your 10mg tablet doesn’t turn into poison after 18 months? Maybe we’re over-engineering this. Simple doesn’t mean unsafe. Just sayin’.
Shelby Ume
December 16 2025
While I appreciate the thoroughness of this breakdown, I must emphasize that the regulatory burden disproportionately impacts small manufacturers and global suppliers. The cost of compliance isn’t just financial-it’s psychological. Many talented teams in developing nations are discouraged from entering the market because they cannot afford the infrastructure required for ICH-compliant testing. We must balance safety with accessibility.
Jade Hovet
December 17 2025
OMG YES!! 😭 I work in pharma QA and this is SO REAL. Last week our stability chamber had a 3.2°C spike for 4 hours. We had to scrap 12 batches. 💀 The FDA doesn’t care if it was a power outage-they just see ‘non-compliance’. Also, emoji for the win: 🧪🌡️📊 #StabilityStruggles
nithin Kuntumadugu
December 18 2025
LOL they say 'stability testing' but everyone knows it's just a racket to keep Indian and Chinese makers out. The brand-name companies pay lobbyists to make sure no one else can compete. And don't get me started on the blockchain thing-like the FDA's gonna stop fraud with a fancy database? They can't even track their own inspectors. #BigPharmaConspiracy 🤫📡
John Fred
December 19 2025
Let’s talk QbD for a sec-this is the future. If you design for stability from the start-material selection, process parameters, packaging-you don’t need 24 months of data. You know it’s stable because you engineered it to be. That’s the paradigm shift. The FDA’s draft guidance is actually forward-thinking. It’s not about more testing-it’s about smarter testing. 🚀
Harriet Wollaston
December 21 2025
I just took a generic lisinopril yesterday. No idea if it passed stability testing. But I’m alive. And so are millions of others. Maybe the system works? Maybe we’re overthinking it? I’m not a scientist, but I trust that someone, somewhere, is checking the numbers so I don’t have to.
Lauren Scrima
December 22 2025
So… you’re telling me we spend $487,500 per ANDA… to prove that a pill doesn’t turn into a science experiment? And we call this ‘medicine’? 🤦♀️👏👏👏
sharon soila
December 22 2025
Every human deserves safe, effective medicine. That’s not a privilege-it’s a right. Stability testing is the bridge between corporate profit and patient safety. We must not lower standards because it’s expensive. We must invest in training, automation, and global equity. This isn’t regulation. This is responsibility.
nina nakamura
December 23 2025
Pathetic. You think this is rigorous? Half these manufacturers are still using paper logs and manual thermometers. The FDA approves 90% of applications despite knowing the data is unreliable. This isn’t science. It’s a performance. You’re all just actors in a play where the audience is too tired to notice the stage is on fire.




Jamie Clark
December 13 2025
Let’s be real-this whole system is a tax on innovation disguised as safety. The FDA doesn’t care if your drug works, they care if your spreadsheet has the right number of decimal places. We’re drowning in paperwork while patients go without because some lab tech in Indiana forgot to log a temperature shift at 3 AM. This isn’t science-it’s bureaucratic theater.