Many people believe that if something is labeled "natural," it must be safer than a pill from the pharmacy. You see it everywhere: herbal teas promising calm, turmeric capsules for inflammation, St. John’s wort for mood support. The message is clear-nature knows best. But here’s the truth: natural doesn’t mean safe. In fact, some of the most dangerous drug interactions happen when people mix herbal supplements with prescription meds-and they have no idea.
Why "Natural" Feels Safer (Even When It’s Not)
The idea that natural equals safe is deeply rooted in culture. It’s not just marketing-it’s psychology. People trust plants because they’ve been used for centuries. They imagine herbal remedies growing wild in forests, untouched by labs or chemicals. But modern supplements aren’t wild herbs. They’re concentrated extracts, often processed in factories, packed into capsules, and sold without oversight. The Dietary Supplement Health and Education Act (DSHEA) of 1994 made this confusion official. Under this law, herbal products are classified as dietary supplements, not drugs. That means companies don’t have to prove they work before selling them. They don’t have to test for interactions with other medications. They don’t even need FDA approval before putting a product on the shelf. All they need is to avoid making false claims about curing diseases. Compare that to pharmaceuticals. Every prescription drug goes through years of clinical trials. The FDA requires proof of safety, dosage accuracy, and potential side effects. Manufacturers must document every step of production. Facilities are inspected. Batch consistency is monitored. Post-market surveillance tracks adverse events. If a drug causes harm, it gets pulled. So why do people think supplements are safer? Because they’re not seeing the same level of scrutiny. And that’s the problem.The Hidden Dangers of Herbal Supplements
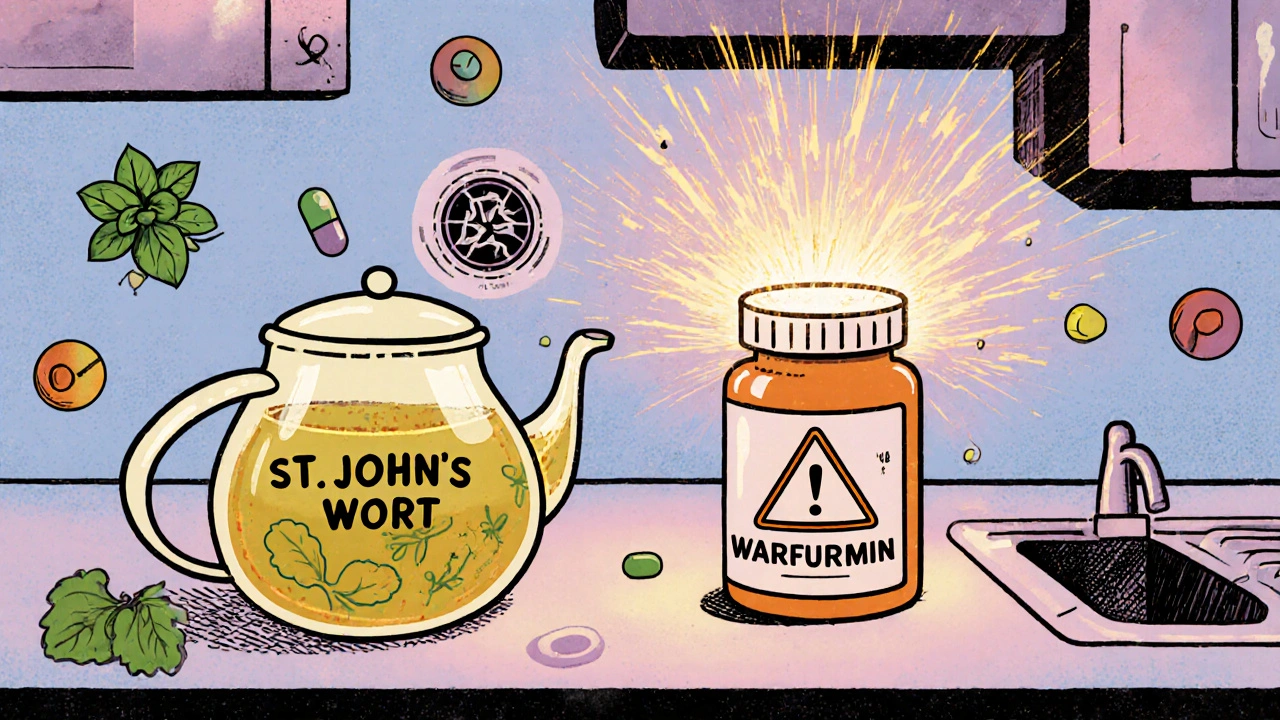
Some natural products are more dangerous than people realize. Take kava. It’s sold as a natural remedy for anxiety. But multiple studies have linked it to severe liver damage. In some cases, people needed transplants. The FDA never banned it, but several countries did. Ephedra is another example. It was once a popular weight-loss supplement. Then people started having heart attacks and strokes. The FDA banned it in 2004. But even after the ban, some products still slipped through-labeled as "herbal energy boosters" or "traditional formulas." Then there’s St. John’s wort. It’s widely promoted for mild depression. But here’s what most users don’t know: it can make birth control pills fail. It can reduce the effectiveness of blood thinners like warfarin. It can interfere with antidepressants, leading to serotonin syndrome-a potentially deadly condition. And it can trigger manic episodes in people with bipolar disorder. Even common herbs like echinacea and ginkgo aren’t risk-free. A large study with over 3,000 older adults found ginkgo did nothing to prevent dementia. Yet millions still take it daily, often alongside blood pressure or diabetes meds. The interaction risk? Real. Ginkgo can thin the blood. When combined with aspirin or clopidogrel, it increases bleeding risk. And let’s not forget foxglove. It’s a wildflower. Its chemical-digoxin-is used in heart medication. But eating the plant? Deadly. People have died from mistaking it for a harmless weed. This isn’t theoretical. It’s documented.Pharmaceuticals Aren’t Perfect-But They’re Tracked
Let’s be fair: pharmaceuticals carry serious risks. The Mayo Clinic reports around 100,000 deaths per year in the U.S. from adverse drug reactions. Opioids cause addiction. Antibiotics trigger C. diff infections. Chemotherapy wipes out healthy cells along with cancerous ones. But here’s the difference: those deaths are counted. They’re reported. They’re studied. When a drug causes harm, the FDA issues warnings. Doctors get alerts. Pharmacies flag interactions. Patients are informed. Supplement side effects? Not so much. The National Poison Control Centers don’t even have a category for herbal reactions. In 2022, there were only 1,200 adverse event reports for dietary supplements. Meanwhile, there were 120,000 for prescription drugs. Is that because supplements are safer? Not likely. Experts say it’s because people don’t report them. They assume natural means harmless. They don’t connect their nausea, headache, or irregular heartbeat to that new turmeric capsule they started last week. And doctors often don’t ask. A 2022 JAMA Internal Medicine study found 70% of patients never tell their doctor they’re taking supplements. That’s a massive blind spot. Imagine taking blood pressure medicine and adding hawthorn berry-another natural heart remedy. Both lower blood pressure. Together? You could crash your pressure to dangerous levels. But if your doctor doesn’t know you’re taking it, they won’t see the cause.
How Supplements Interact With Medications
The body doesn’t care if a compound comes from a plant or a lab. It reacts to the chemical. And many herbal supplements affect the same liver enzymes that process prescription drugs. The CYP450 enzyme system is the main pathway for drug metabolism. St. John’s wort, grapefruit juice, goldenseal, and even some green tea extracts can interfere with it. Here’s what happens:- Speed up metabolism: The supplement tells your liver to break down the drug faster. Result? The drug doesn’t work. Birth control fails. Antidepressants lose effect. Transplant rejection can occur.
- Slow down metabolism: The supplement blocks the liver from processing the drug. Result? Toxic buildup. Blood thinners become dangerous. Cholesterol meds cause muscle damage. Painkillers overload your system.
The Regulatory Gap
The U.S. dietary supplement market is worth $50 billion. The pharmaceutical market? $600 billion. And yet, the FDA spends a fraction of its resources on supplements. In 2023, the FDA issued just 35 warning letters to supplement manufacturers. Thousands of products with unsafe ingredients-like hidden steroids, stimulants, or unapproved drugs-still hit shelves. Why? Because the agency can’t test everything. They rely on consumer complaints and rare lab findings. Meanwhile, pharmaceutical companies spend $100 billion a year on R&D. They’re held to standards that require them to prove safety before sale. Supplements? They’re on the market before anyone checks. The Dietary Supplement Listing Act of 2023, currently in Congress, could change that. It proposes requiring pre-market safety reviews for new supplements. But until then, the burden is on the consumer.
What You Can Do to Stay Safe
You don’t have to give up natural products. But you do need to treat them like medicine.- Always tell your doctor what you’re taking. Even if it’s "just a supplement." Write it down. Bring the bottle.
- Look for third-party verification. Products with the USP Verified Mark have been tested for purity, potency, and contaminants. Only 15% of major brands have it-but it’s worth choosing.
- Don’t assume "all-natural" means safe. Read labels. Research ingredients. Check the NIH Office of Dietary Supplements website for evidence-based info.
- Be skeptical of bold claims. "Cures arthritis!" "Boosts immunity!" If it sounds too good to be true, it is.
- Stop taking supplements before surgery. Many herbs increase bleeding risk. Your surgeon needs to know.
Bottom Line: Safety Isn’t About Natural or Synthetic
The real question isn’t whether natural products are safer than pharmaceuticals. It’s whether you’re using them wisely. Natural doesn’t mean harmless. Synthetic doesn’t mean evil. What matters is quality, dosage, and interaction risk. A poorly made herbal supplement can be just as dangerous as a misused prescription. And a well-regulated pharmaceutical, taken correctly, can save your life. The truth is simple: if you’re taking anything to change how your body works-whether it’s a pill, a tea, or a capsule-you’re taking medicine. Treat it like it.Are herbal supplements regulated like prescription drugs?
No. Herbal supplements are classified as dietary supplements under DSHEA, not drugs. That means they don’t need FDA approval before sale, don’t require proof of effectiveness, and aren’t tested for interactions with medications. Prescription drugs, by contrast, must go through years of clinical trials and ongoing safety monitoring.
Can natural supplements interact with my medications?
Yes, and often dangerously. St. John’s wort can reduce the effectiveness of birth control, antidepressants, and blood thinners. Ginkgo can increase bleeding risk when taken with aspirin or warfarin. Even common vitamins like E and K can interfere with medications. These interactions are real, documented, and often overlooked.
Why don’t supplement labels warn about drug interactions?
Because they’re not required to. Unlike prescription drugs, which must include detailed interaction warnings, supplement manufacturers aren’t obligated to test for or disclose interactions. Many don’t even know what their product does in combination with other substances.
Is it safe to take supplements if I’m healthy?
Not necessarily. Even healthy people can have bad reactions-especially if they’re taking multiple supplements or suddenly start a new one. High doses of certain vitamins or herbs can stress the liver, affect blood pressure, or trigger allergic reactions. What’s "safe" for one person might be harmful to another.
How do I know if a supplement is high quality?
Look for the USP Verified Mark on the label. It means an independent lab tested the product for purity, potency, and absence of harmful contaminants. Other trusted seals include NSF International and ConsumerLab.com. Avoid products with vague claims like "all-natural" or "proprietary blend"-those often hide ingredients.
Should I stop taking supplements before surgery?
Yes. Many supplements-like ginkgo, garlic, ginger, and fish oil-can increase bleeding risk. Others, like kava or valerian, can interfere with anesthesia. Always tell your surgeon what you’re taking and follow their advice on when to stop.
13 Comments
Nicola Mari
November 28 2025
People who think herbal supplements are safe are either dangerously naive or willfully ignorant. This isn’t yoga and crystals-it’s pharmacology with a prettier label. You wouldn’t drink bleach because it’s 'natural,' so why do this?
Sam txf
November 30 2025
Let me break this down for the brain-dead: if your liver has to process a compound, it doesn’t give a damn if it came from a tree or a chemist’s beaker. St. John’s wort is a CYP450 saboteur-period. Stop treating your body like a DIY bio-lab.
George Hook
December 1 2025
I’ve been a nurse for 27 years, and I’ve seen too many patients come in with liver enzymes through the roof because they were taking ‘just a little turmeric’ with their warfarin. The problem isn’t that supplements are inherently evil-it’s that nobody teaches people how to use them responsibly. We treat them like candy, not medicine. And then we’re shocked when things go wrong. The system fails us by not requiring clear labeling, but the real failure is our cultural laziness-thinking ‘natural’ means ‘no consequences.’
jaya sreeraagam
December 2 2025
I live in India where ayurveda has been used for thousands of years, and I can tell you-when done right, with proper guidance, it’s powerful. But modern capsules? They’re not ayurveda. They’re chemical extracts in plastic bottles with no lineage. Please don’t equate tradition with mass-market supplements. Talk to a qualified practitioner, not a Walmart shelf. And yes, I’ve seen people on blood thinners take ashwagandha and almost crash. It’s not magic-it’s medicine. Treat it that way.
Katrina Sofiya
December 2 2025
Thank you for writing this with such clarity. So many people are terrified of pharmaceuticals and don’t realize that the real danger is ignorance-not the pill itself. Knowledge is power, and you’ve given us the tools to be informed. Please keep speaking up-this message needs to reach more people.
kaushik dutta
December 3 2025
The CYP450 system is the linchpin here. When you introduce a flavonoid-rich extract like goldenseal or grapefruit juice into the hepatic metabolic cascade, you’re essentially hijacking phase I enzymatic detoxification pathways. This isn’t anecdotal-it’s biochemically deterministic. The FDA’s regulatory gap isn’t a loophole-it’s a systemic failure of risk stratification in a neoliberal health economy. You’re not just taking a supplement; you’re engaging in a pharmacokinetic gamble with your cytochrome P450 isoforms.
doug schlenker
December 5 2025
I get why people turn to supplements. I’ve been on antidepressants for years and I get tired of the side effects. But I also know that if I’m going to try something like St. John’s wort, I need to talk to my doctor first. I did. He told me it could interfere with my meds. So I didn’t. Simple. No drama. Just responsibility. We don’t need fear-we need better communication.
Olivia Gracelynn Starsmith
December 7 2025
I used to take ginkgo because I thought it would help my memory but after reading this I stopped immediately and told my doctor. He said I was lucky I didn’t combine it with my blood pressure med. I never thought about interactions because I assumed natural = harmless. Big mistake. Lesson learned
Skye Hamilton
December 8 2025
So... you're saying the FDA is the good guy now? Funny. They let OxyContin through. They let vaping products kill kids. They approved those damn fen-phen pills. You think they care about you? They care about profits. This post is just corporate propaganda dressed up as concern.
Maria Romina Aguilar
December 9 2025
I’m not saying supplements are safe... but... maybe... just maybe... the pharmaceutical industry is exaggerating the risks to maintain control? I mean... think about it... they spend billions on advertising... and then they make you feel guilty for wanting something... natural... and... well... I just... I don’t know...
Brandon Trevino
December 10 2025
The data is unambiguous. Supplement adverse event reporting is underreported by a factor of 100. The FDA’s 35 warning letters in 2023 reflect resource constraints, not regulatory efficacy. Meanwhile, the USP Verified Mark is the only credible metric for quality, and less than 15% of products meet it. This is not a debate. It’s a public health emergency masked as consumer choice.
Denise Wiley
December 12 2025
I love that you included the USP tip. I just started checking for it after my mom had a bad reaction to a ‘natural’ sleep aid. She didn’t even know it had melatonin and valerian mixed with a hidden stimulant. Now I always bring the bottle to appointments. It’s not about fear-it’s about being prepared. Thank you for the clarity.





Austin Simko
November 28 2025
FDA is rigged. They take bribes from Big Pharma to keep natural remedies off the shelves. You think they don’t know kava helps anxiety? They just don’t want you curing yourself.